Methodological advances enabled by the construction of a synthetic yeast genome
- PMID: 38653205
- PMCID: PMC11046031
- DOI: 10.1016/j.crmeth.2024.100761
Methodological advances enabled by the construction of a synthetic yeast genome
Abstract
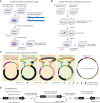
The international Synthetic Yeast Project (Sc2.0) aims to construct the first synthetic designer eukaryote genome. Over the past few years, the Sc2.0 consortium has achieved several significant milestones by synthesizing and characterizing all 16 nuclear chromosomes of the yeast Saccharomyces cerevisiae, as well as a 17thde novo neochromosome containing all nuclear tRNA genes. In this commentary, we discuss the recent technological advances achieved in this project and provide a perspective on how they will impact the emerging field of synthetic genomics in the future.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
The Fifth Annual Sc2.0 and Synthetic Genomes Conference: Synthetic Genomes in High Gear.ACS Synth Biol. 2016 Sep 16;5(9):920-2. doi: 10.1021/acssynbio.6b00227. ACS Synth Biol. 2016. PMID: 27633830 Review.
-
Design, construction, and functional characterization of a tRNA neochromosome in yeast.Cell. 2023 Nov 22;186(24):5237-5253.e22. doi: 10.1016/j.cell.2023.10.015. Epub 2023 Nov 8. Cell. 2023. PMID: 37944512
-
Synthetic chromosome arms function in yeast and generate phenotypic diversity by design.Nature. 2011 Sep 14;477(7365):471-6. doi: 10.1038/nature10403. Nature. 2011. PMID: 21918511 Free PMC article.
-
Design of a synthetic yeast genome.Science. 2017 Mar 10;355(6329):1040-1044. doi: 10.1126/science.aaf4557. Science. 2017. PMID: 28280199
-
Synthetic biology stretching the realms of possibility in wine yeast research.Int J Food Microbiol. 2017 Jul 3;252:24-34. doi: 10.1016/j.ijfoodmicro.2017.04.006. Epub 2017 Apr 20. Int J Food Microbiol. 2017. PMID: 28458189 Review.
Cited by
-
Genetic engineering in diatoms: advances and prospects.Plant J. 2025 Mar;121(6):e70102. doi: 10.1111/tpj.70102. Plant J. 2025. PMID: 40089910 Free PMC article. Review.
-
The Transformation Experiment of Frederick Griffith II: Inclusion of Cellular Heredity for the Creation of Novel Microorganisms.Bioengineering (Basel). 2025 May 15;12(5):532. doi: 10.3390/bioengineering12050532. Bioengineering (Basel). 2025. PMID: 40428151 Free PMC article. Review.
-
Toward a Quadruplet Codon Mitochondrial Genetic Code.ACS Synth Biol. 2024 Dec 20;13(12):4175-4179. doi: 10.1021/acssynbio.4c00630. Epub 2024 Dec 4. ACS Synth Biol. 2024. PMID: 39631441 Free PMC article.
-
The Transformation Experiment of Frederick Griffith I: Its Narrowing and Potential for the Creation of Novel Microorganisms.Bioengineering (Basel). 2025 Mar 20;12(3):324. doi: 10.3390/bioengineering12030324. Bioengineering (Basel). 2025. PMID: 40150788 Free PMC article. Review.
-
Iterative SCRaMbLE for engineering synthetic genome modules and chromosomes.Nat Commun. 2025 Aug 7;16(1):7278. doi: 10.1038/s41467-025-62356-y. Nat Commun. 2025. PMID: 40774952 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

