Nitrogen and Nod factor signaling determine Lotus japonicus root exudate composition and bacterial assembly
- PMID: 38653767
- PMCID: PMC11039659
- DOI: 10.1038/s41467-024-47752-0
Nitrogen and Nod factor signaling determine Lotus japonicus root exudate composition and bacterial assembly
Abstract
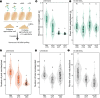
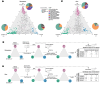
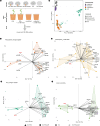
Symbiosis with soil-dwelling bacteria that fix atmospheric nitrogen allows legume plants to grow in nitrogen-depleted soil. Symbiosis impacts the assembly of root microbiota, but it is unknown how the interaction between the legume host and rhizobia impacts the remaining microbiota and whether it depends on nitrogen nutrition. Here, we use plant and bacterial mutants to address the role of Nod factor signaling on Lotus japonicus root microbiota assembly. We find that Nod factors are produced by symbionts to activate Nod factor signaling in the host and that this modulates the root exudate profile and the assembly of a symbiotic root microbiota. Lotus plants with different symbiotic abilities, grown in unfertilized or nitrate-supplemented soils, display three nitrogen-dependent nutritional states: starved, symbiotic, or inorganic. We find that root and rhizosphere microbiomes associated with these states differ in composition and connectivity, demonstrating that symbiosis and inorganic nitrogen impact the legume root microbiota differently. Finally, we demonstrate that selected bacterial genera characterizing state-dependent microbiomes have a high level of accurate prediction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

