Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing
- PMID: 38653959
- PMCID: PMC11039765
- DOI: 10.1038/s41467-024-47696-5
Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing
Abstract
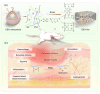
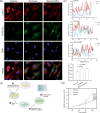
Wound healing is an obvious clinical concern that can be hindered by inadequate angiogenesis, inflammation, and chronic hypoxia. While exosomes derived from adipose tissue-derived stem cells have shown promise in accelerating healing by carrying therapeutic growth factors and microRNAs, intracellular cargo delivery is compromised in hypoxic tissues due to activated hypoxia-induced endocytic recycling. To address this challenge, we have developed a strategy to coat oxygen nanobubbles with exosomes and incorporate them into a polyvinyl alcohol/gelatin hybrid hydrogel. This approach not only alleviates wound hypoxia but also offers an efficient means of delivering exosome-coated nanoparticles in hypoxic conditions. The self-healing properties of the hydrogel, along with its component, gelatin, aids in hemostasis, while its crosslinking bonds facilitate hydrogen peroxide decomposition, to ameliorate wound inflammation. Here, we show the potential of this multifunctional hydrogel for enhanced healing, promoting angiogenesis, facilitating exosome delivery, mitigating hypoxia, and inhibiting inflammation in a male rat full-thickness wound model.
© 2024. The Author(s).
Conflict of interest statement
J.I. and X.H. filed a provisional patent application for the exosome-coated oxygen nanobubble-laden hydrogel (U.S. Patent Application No.: 63/631,255). The remaining authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

