Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: a prospective, single arm, phase I trial
- PMID: 38653983
- PMCID: PMC11039759
- DOI: 10.1038/s41392-024-01793-5
Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: a prospective, single arm, phase I trial
Abstract
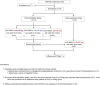
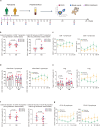
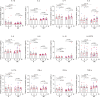
Patients with refractory immune thrombocytopenia (ITP) frequently encounter substantial bleeding risks and demonstrate limited responsiveness to existing therapies. Umbilical cord-derived mesenchymal stem cells (UC-MSCs) present a promising alternative, capitalizing on their low immunogenicity and potent immunomodulatory effects for treating diverse autoimmune disorders. This prospective phase I trial enrolled eighteen eligible patients to explore the safety and efficacy of UC-MSCs in treating refractory ITP. The research design included administering UC-MSCs at escalating doses of 0.5 × 106 cells/kg, 1.0 × 106 cells/kg, and 2.0 × 106 cells/kg weekly for four consecutive weeks across three cohorts during the dose-escalation phase, followed by a dose of 2.0 × 106 cells/kg weekly for the dose-expansion phase. Adverse events, platelet counts, and changes in peripheral blood immunity were monitored and recorded throughout the administration and follow-up period. Ultimately, 12 (with an addition of three patients in the 2.0 × 106 cells/kg group due to dose-limiting toxicity) and six patients were enrolled in the dose-escalation and dose-expansion phase, respectively. Thirteen patients (13/18, 72.2%) experienced one or more treatment emergent adverse events. Serious adverse events occurred in four patients (4/18, 22.2%), including gastrointestinal hemorrhage (2/4), profuse menstruation (1/4), and acute myocardial infarction (1/4). The response rates were 41.7% in the dose-escalation phase (5/12, two received 1.0 × 106 cells/kg per week, and three received 2.0 × 106 cells/kg per week) and 50.0% (3/6) in the dose-expansion phase. The overall response rate was 44.4% (8/18) among all enrolled patients. To sum up, UC-MSCs are effective and well tolerated in treating refractory ITP (ClinicalTrials.gov ID: NCT04014166).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
- 82270152/National Natural Science Foundation of China (National Science Foundation of China)
- 81970121/National Natural Science Foundation of China (National Science Foundation of China)
- 82100151/National Natural Science Foundation of China (National Science Foundation of China)
- 82070125/National Natural Science Foundation of China (National Science Foundation of China)
- 82170127/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical

