The gut microbiome-prostate cancer crosstalk is modulated by dietary polyunsaturated long-chain fatty acids
- PMID: 38654015
- PMCID: PMC11039720
- DOI: 10.1038/s41467-024-45332-w
The gut microbiome-prostate cancer crosstalk is modulated by dietary polyunsaturated long-chain fatty acids
Abstract
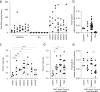
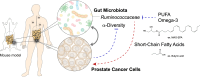
The gut microbiota modulates response to hormonal treatments in prostate cancer (PCa) patients, but whether it influences PCa progression remains unknown. Here, we show a reduction in fecal microbiota alpha-diversity correlating with increase tumour burden in two distinct groups of hormonotherapy naïve PCa patients and three murine PCa models. Fecal microbiota transplantation (FMT) from patients with high PCa volume is sufficient to stimulate the growth of mouse PCa revealing the existence of a gut microbiome-cancer crosstalk. Analysis of gut microbial-related pathways in mice with aggressive PCa identifies three enzymes responsible for the metabolism of long-chain fatty acids (LCFA). Supplementation with LCFA omega-3 MAG-EPA is sufficient to reduce PCa growth in mice and cancer up-grading in pre-prostatectomy PCa patients correlating with a reduction of gut Ruminococcaceae in both and fecal butyrate levels in PCa patients. This suggests that the beneficial effect of omega-3 rich diet is mediated in part by modulating the crosstalk between gut microbes and their metabolites in men with PCa.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

