CircCDYL2 bolsters radiotherapy resistance in nasopharyngeal carcinoma by promoting RAD51 translation initiation for enhanced homologous recombination repair
- PMID: 38654320
- PMCID: PMC11036759
- DOI: 10.1186/s13046-024-03049-0
CircCDYL2 bolsters radiotherapy resistance in nasopharyngeal carcinoma by promoting RAD51 translation initiation for enhanced homologous recombination repair
Abstract
Background: Radiation therapy stands to be one of the primary approaches in the clinical treatment of malignant tumors. Nasopharyngeal Carcinoma, a malignancy predominantly treated with radiation therapy, provides an invaluable model for investigating the mechanisms underlying radiation therapy resistance in cancer. While some reports have suggested the involvement of circRNAs in modulating resistance to radiation therapy, the underpinning mechanisms remain unclear.
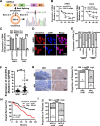
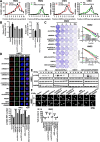
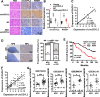
Methods: RT-qPCR and in situ hybridization were used to detect the expression level of circCDYL2 in nasopharyngeal carcinoma tissue samples. The effect of circCDYL2 on radiotherapy resistance in nasopharyngeal carcinoma was demonstrated by in vitro and in vivo functional experiments. The HR-GFP reporter assay determined that circCDYL2 affected homologous recombination repair. RNA pull down, RIP, western blotting, IF, and polysome profiling assays were used to verify that circCDYL2 promoted the translation of RAD51 by binding to EIF3D protein.
Results: We have identified circCDYL2 as highly expressed in nasopharyngeal carcinoma tissues, and it was closely associated with poor prognosis. In vitro and in vivo experiments demonstrate that circCDYL2 plays a pivotal role in promoting radiotherapy resistance in nasopharyngeal carcinoma. Our investigation unveils a specific mechanism by which circCDYL2, acting as a scaffold molecule, recruits eukaryotic translation initiation factor 3 subunit D protein (EIF3D) to the 5'-UTR of RAD51 mRNA, a crucial component of the DNA damage repair pathway to facilitate the initiation of RAD51 translation and enhance homologous recombination repair capability, and ultimately leads to radiotherapy resistance in nasopharyngeal carcinoma.
Conclusions: These findings establish a novel role of the circCDYL2/EIF3D/RAD51 axis in nasopharyngeal carcinoma radiotherapy resistance. Our work not only sheds light on the underlying molecular mechanism but also highlights the potential of circCDYL2 as a therapeutic sensitization target and a promising prognostic molecular marker for nasopharyngeal carcinoma.
Keywords: Homologous recombination repair; Nasopharyngeal carcinoma; RAD51; Radiotherapy resistance; circCDYL2.
© 2024. The Author(s).
Conflict of interest statement
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication.
The authors declare that they have no conflict of interest.
Figures




References
-
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

