Tumor-suppressive miR-4732-3p is sorted into fucosylated exosome by hnRNPK to avoid the inhibition of lung cancer progression
- PMID: 38654325
- PMCID: PMC11036635
- DOI: 10.1186/s13046-024-03048-1
Tumor-suppressive miR-4732-3p is sorted into fucosylated exosome by hnRNPK to avoid the inhibition of lung cancer progression
Abstract
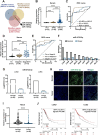
Background: Aberrant fucosylation observed in cancer cells contributes to an augmented release of fucosylated exosomes into the bloodstream, where miRNAs including miR-4732-3p hold promise as potential tumor biomarkers in our pilot study. However, the mechanisms underlying the sorting of miR-4732-3p into fucosylated exosomes during lung cancer progression remain poorly understood.
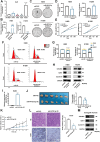
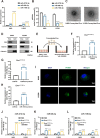
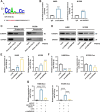
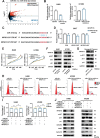
Methods: A fucose-captured strategy based on lentil lectin-magnetic beads was utilized to isolate fucosylated exosomes and evaluate the efficiency for capturing tumor-derived exosomes using nanoparticle tracking analysis (NTA). Fluorescence in situ hybridization (FISH) and qRT-PCR were performed to determine the levels of miR-4732-3p in non-small cell lung cancer (NSCLC) tissue samples. A co-culture system was established to assess the release of miRNA via exosomes from NSCLC cells. RNA immunoprecipitation (RIP) and miRNA pull-down were applied to validate the interaction between miR-4732-3p and heterogeneous nuclear ribonucleoprotein K (hnRNPK) protein. Cell functional assays, cell derived xenograft, dual-luciferase reporter experiments, and western blot were applied to examine the effects of miR-4732-3p on MFSD12 and its downstream signaling pathways, and the impact of hnRNPK in NSCLC.
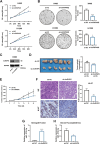
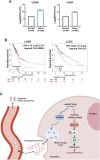
Results: We enriched exosomes derived from NSCLC cells using the fucose-captured strategy and detected a significant upregulation of miR-4732-3p in fucosylated exosomes present in the serum, while its expression declined in NSCLC tissues. miR-4732-3p functioned as a tumor suppressor in NSCLC by targeting 3'UTR of MFSD12, thereby inhibiting AKT/p21 signaling pathway to induce cell cycle arrest in G2/M phase. NSCLC cells preferentially released miR-4732-3p via exosomes instead of retaining them intracellularly, which was facilitated by the interaction of miR-4732-3p with hnRNPK protein for selective sorting into fucosylated exosomes. Moreover, knockdown of hnRNPK suppressed NSCLC cell proliferation, with the elevated levels of miR-4732-3p in NSCLC tissues but the decreased expression in serum fucosylated exosomes.
Conclusions: NSCLC cells escape suppressive effects of miR-4732-3p through hnRNPK-mediated sorting of them into fucosylated exosomes, thus supporting cell malignant properties and promoting NSCLC progression. Our study provides a promising biomarker for NSCLC and opens a novel avenue for NSCLC therapy by targeting hnRNPK to prevent the "exosome escape" of tumor-suppressive miR-4732-3p from NSCLC cells.
Keywords: Exosome escape; Fucosylated exosome; MFSD12; hnRNPK; miR-4732-3p.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
Similar articles
-
Downregulation of N-Acetylglucosaminyltransferase GCNT3 by miR-302b-3p Decreases Non-Small Cell Lung Cancer (NSCLC) Cell Proliferation, Migration and Invasion.Cell Physiol Biochem. 2018;50(3):987-1004. doi: 10.1159/000494482. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. PMID: 30355927
-
Serum exosomal miR-454-3p contributes to malignant progression of lung cancer by inhibiting HHEX.Mol Cell Probes. 2025 Apr;80:102019. doi: 10.1016/j.mcp.2025.102019. Epub 2025 Feb 16. Mol Cell Probes. 2025. PMID: 39929349
-
LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis.Thorac Cancer. 2020 Mar;11(3):549-560. doi: 10.1111/1759-7714.13280. Epub 2020 Jan 10. Thorac Cancer. 2020. PMID: 31923353 Free PMC article.
-
Small extracellular vesicles: Multi-functional aspects in non-small cell lung carcinoma.Crit Rev Oncol Hematol. 2024 Jun;198:104341. doi: 10.1016/j.critrevonc.2024.104341. Epub 2024 Apr 3. Crit Rev Oncol Hematol. 2024. PMID: 38575042 Review.
-
The potential of tumor-derived exosomes for noninvasive cancer monitoring.Expert Rev Mol Diagn. 2015;15(10):1293-310. doi: 10.1586/14737159.2015.1071666. Epub 2015 Aug 2. Expert Rev Mol Diagn. 2015. PMID: 26289602 Free PMC article. Review.
Cited by
-
The long noncoding RNA MIR4435-2HG enhances the migration, promotion, and glycolysis of nonsmall cell lung cancer cells by targeting the miR-371a-5p/SOX2/PI3K/Akt axis.SAGE Open Med. 2024 Nov 8;12:20503121241289290. doi: 10.1177/20503121241289290. eCollection 2024. SAGE Open Med. 2024. PMID: 39526092 Free PMC article.
-
FUCA1: An Underexplored p53 Target Gene Linking Glycosylation and Cancer Progression.Cancers (Basel). 2024 Aug 2;16(15):2753. doi: 10.3390/cancers16152753. Cancers (Basel). 2024. PMID: 39123480 Free PMC article. Review.
-
circNRIP1 impairs tumorigenesis of colorectal cancer by sponging IGF2BP1 and decreasing NACC1 mRNA stability.Gastroenterol Rep (Oxf). 2025 Jun 20;13:goaf041. doi: 10.1093/gastro/goaf041. eCollection 2025. Gastroenterol Rep (Oxf). 2025. PMID: 40584151 Free PMC article.
-
Emerging roles of exosomal circRNAs in non-small cell lung cancer.J Transl Med. 2025 Apr 30;23(1):490. doi: 10.1186/s12967-025-06463-w. J Transl Med. 2025. PMID: 40307927 Free PMC article. Review.
-
Harnessing EVs-ncRNA for Lung Cancer: From Oncogenic Pathways to Novel Diagnostic and Therapeutic Strategies.Int J Nanomedicine. 2025 Jul 14;20:9031-9054. doi: 10.2147/IJN.S528115. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40689018 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

