Regulation of human microglial gene expression and function via RNAase-H active antisense oligonucleotides in vivo in Alzheimer's disease
- PMID: 38654375
- PMCID: PMC11040766
- DOI: 10.1186/s13024-024-00725-9
Regulation of human microglial gene expression and function via RNAase-H active antisense oligonucleotides in vivo in Alzheimer's disease
Abstract
Background: Microglia play important roles in maintaining brain homeostasis and neurodegeneration. The discovery of genetic variants in genes predominately or exclusively expressed in myeloid cells, such as Apolipoprotein E (APOE) and triggering receptor expressed on myeloid cells 2 (TREM2), as the strongest risk factors for Alzheimer's disease (AD) highlights the importance of microglial biology in the brain. The sequence, structure and function of several microglial proteins are poorly conserved across species, which has hampered the development of strategies aiming to modulate the expression of specific microglial genes. One way to target APOE and TREM2 is to modulate their expression using antisense oligonucleotides (ASOs).
Methods: In this study, we identified, produced, and tested novel, selective and potent ASOs for human APOE and TREM2. We used a combination of in vitro iPSC-microglia models, as well as microglial xenotransplanted mice to provide proof of activity in human microglial in vivo.
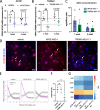
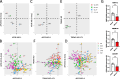
Results: We proved their efficacy in human iPSC microglia in vitro, as well as their pharmacological activity in vivo in a xenografted microglia model. We demonstrate ASOs targeting human microglia can modify their transcriptional profile and their response to amyloid-β plaques in vivo in a model of AD.
Conclusions: This study is the first proof-of-concept that human microglial can be modulated using ASOs in a dose-dependent manner to manipulate microglia phenotypes and response to neurodegeneration in vivo.
Keywords: APOE; Alzheimer’s disease; Antisense oligonucleotide; Microglia; Neuroinflammation; TREM2.
© 2024. The Author(s).
Conflict of interest statement
L.V., A.N., M.K., A.D.B., I.V.D.W. and C.D.Y. are employees of Janssen Pharmaceutica, pharmaceutical companies of Johnson&Johnson. I.G. and A.L. are employees of Muna Therapeutics since October 2022. B.D.S. is or has been a consultant for Eli Lilly, Biogen, Janssen Pharmaceutica, Eisai, AbbVie and other companies. B.D.S is also a scientific founder of Augustine Therapeutics and a scientific founder and stockholder of Muna Therapeutics. R.M. has scientific collaborations with Janssen Pharmaceutica, Alector and Nodthera and is consultant of Sanofi.
Figures




Similar articles
-
Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer's disease.Nat Commun. 2020 Oct 23;11(1):5370. doi: 10.1038/s41467-020-19227-5. Nat Commun. 2020. PMID: 33097708 Free PMC article.
-
The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases.Immunity. 2017 Sep 19;47(3):566-581.e9. doi: 10.1016/j.immuni.2017.08.008. Immunity. 2017. PMID: 28930663 Free PMC article.
-
TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway.J Neurosci. 2017 Feb 15;37(7):1772-1784. doi: 10.1523/JNEUROSCI.2459-16.2017. Epub 2017 Jan 11. J Neurosci. 2017. PMID: 28077724 Free PMC article.
-
The Role of APOE and TREM2 in Alzheimer's Disease-Current Understanding and Perspectives.Int J Mol Sci. 2018 Dec 26;20(1):81. doi: 10.3390/ijms20010081. Int J Mol Sci. 2018. PMID: 30587772 Free PMC article. Review.
-
TREM2, microglia, and Alzheimer's disease.Mech Ageing Dev. 2021 Apr;195:111438. doi: 10.1016/j.mad.2021.111438. Epub 2021 Jan 28. Mech Ageing Dev. 2021. PMID: 33516818 Review.
Cited by
-
Decoding microglial functions in Alzheimer's disease: insights from human models.Trends Immunol. 2025 Apr;46(4):310-323. doi: 10.1016/j.it.2025.02.011. Epub 2025 Mar 19. Trends Immunol. 2025. PMID: 40113535 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

