A generalizable data-driven model of atrophy heterogeneity and progression in memory clinic settings
- PMID: 38654513
- PMCID: PMC11224599
- DOI: 10.1093/brain/awae118
A generalizable data-driven model of atrophy heterogeneity and progression in memory clinic settings
Abstract
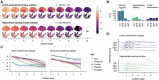
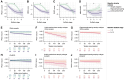
Memory clinic patients are a heterogeneous population representing various aetiologies of pathological ageing. It is not known whether divergent spatiotemporal progression patterns of brain atrophy, as previously described in Alzheimer's disease patients, are prevalent and clinically meaningful in this group of older adults. To uncover distinct atrophy subtypes, we applied the Subtype and Stage Inference (SuStaIn) algorithm to baseline structural MRI data from 813 participants enrolled in the DELCODE cohort (mean ± standard deviation, age = 70.67 ± 6.07 years, 52% females). Participants were cognitively unimpaired (n = 285) or fulfilled diagnostic criteria for subjective cognitive decline (n = 342), mild cognitive impairment (n = 118) or dementia of the Alzheimer's type (n = 68). Atrophy subtypes were compared in baseline demographics, fluid Alzheimer's disease biomarker levels, the Preclinical Alzheimer Cognitive Composite (PACC-5) as well as episodic memory and executive functioning. PACC-5 trajectories over up to 240 weeks were examined. To test whether baseline atrophy subtype and stage predicted clinical trajectories before manifest cognitive impairment, we analysed PACC-5 trajectories and mild cognitive impairment conversion rates of cognitively unimpaired participants and those with subjective cognitive decline. Limbic-predominant and hippocampal-sparing atrophy subtypes were identified. Limbic-predominant atrophy initially affected the medial temporal lobes, followed by further temporal regions and, finally, the remaining cortical regions. At baseline, this subtype was related to older age, more pathological Alzheimer's disease biomarker levels, APOE ε4 carriership and an amnestic cognitive impairment. Hippocampal-sparing atrophy initially occurred outside the temporal lobe, with the medial temporal lobe spared up to advanced atrophy stages. This atrophy pattern also affected individuals with positive Alzheimer's disease biomarkers and was associated with more generalized cognitive impairment. Limbic-predominant atrophy, in all participants and in only unimpaired participants, was linked to more negative longitudinal PACC-5 slopes than observed in participants without or with hippocampal-sparing atrophy and increased the risk of mild cognitive impairment conversion. SuStaIn modelling was repeated in a sample from the Swedish BioFINDER-2 cohort. Highly similar atrophy progression patterns and associated cognitive profiles were identified. Cross-cohort model generalizability, at both the subject and the group level, was excellent, indicating reliable performance in previously unseen data. The proposed model is a promising tool for capturing heterogeneity among older adults at early at-risk states for Alzheimer's disease in applied settings. The implementation of atrophy subtype- and stage-specific end points might increase the statistical power of pharmacological trials targeting early Alzheimer's disease.
Keywords: Alzheimer’s disease; disease heterogeneity; episodic memory; executive function; structural MRI.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
S.P. has acquired research support (for the institution) from ki elements/ADDF and Avid. In the past 2 years, he has received consultancy/speaker fees from Bioartic, Biogen, Esai, Lilly and Roche. E.D. reports personal fees from Biogen, Roche, Lilly, Eisai and UCL Consultancy, in addition to non-financial support from Rox Health. D.B. and E.D. are scientific co-founders of neotiv GmbH and own company shares. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens.
Figures



References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

