This is a preprint.
IL-10 dependent adaptation allows macrophages to adjust inflammatory responses to TLR4 stimulation history
- PMID: 38654826
- PMCID: PMC11037870
- DOI: 10.1101/2024.03.28.587272
IL-10 dependent adaptation allows macrophages to adjust inflammatory responses to TLR4 stimulation history
Abstract
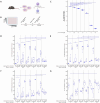
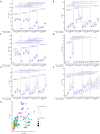
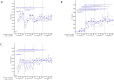
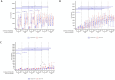
During an infection, innate immune cells must adjust nature and strength of their responses to changing pathogen abundances. To determine how stimulation of the pathogen sensing TLR4 shapes subsequent macrophage responses, we systematically varied priming and restimulation concentrations of its ligand KLA. We find that different priming strengths have very distinct effects at multiple stages of the signaling response, including receptor internalization, MAPK activation, cytokine and chemokine production, and nuclear translocation and chromatin association of NFκB and IκB members. In particular, restimulation-induced TNF-α production required KLA doses equal to or greater than those used for prior exposure, indicating that macrophages can detect and adaptively respond to changing TLR4 stimuli. Interestingly, while such adaptation was dependent on the anti-inflammatory cytokine IL-10, exogenous concentrations of IL-10 corresponding to those secreted after strong priming did not exert suppressive effects on TNF-α without such prior priming, confirming the critical role of TLR4 stimulation history.
Keywords: IL-10; adaptation; inflammation; innate immunity; macrophages; pattern recognition receptor (PRR); temporal gradients; toll-like receptor 4 (TLR4).
Figures


Similar articles
-
Cell-free hemoglobin triggers macrophage cytokine production via TLR4 and MyD88.Am J Physiol Lung Cell Mol Physiol. 2024 Jan 1;326(1):L29-L38. doi: 10.1152/ajplung.00123.2023. Epub 2023 Nov 22. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 37991487 Free PMC article.
-
Activation of Toll-like receptors by Burkholderia pseudomallei.BMC Immunol. 2008 Aug 8;9:46. doi: 10.1186/1471-2172-9-46. BMC Immunol. 2008. PMID: 18691413 Free PMC article.
-
Inhibition of specific signaling pathways rather than epigenetic silencing of effector genes is the leading mechanism of innate tolerance.Front Immunol. 2023 Jan 26;14:1006002. doi: 10.3389/fimmu.2023.1006002. eCollection 2023. Front Immunol. 2023. PMID: 36776861 Free PMC article.
-
Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
References
-
- Medzhitov R., Toll-like receptors and innate immunity. Nat Rev Immunol, 2001. 1(2): p. 135–45. - PubMed
-
- Lu Y.C., Yeh W.C., and Ohashi P.S., LPS/TLR4 signal transduction pathway. Cytokine, 2008. 42(2): p. 145–151. - PubMed
-
- Gustafson G.L., Rhodes M.J., and Hegel T., Monophosphoryl lipid A as a prophylactic for sepsis and septic shock. Prog Clin Biol Res, 1995. 392: p. 567–79. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
