Measurement of Neuropeptide Y in Aptamer-Modified Planar Electrodes
- PMID: 38654828
- PMCID: PMC11034791
- DOI: 10.1016/j.electacta.2024.144243
Measurement of Neuropeptide Y in Aptamer-Modified Planar Electrodes
Abstract
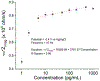
Electrochemical impedance spectroscopy (EIS) is a powerful technique for studying the interaction at electrode/solution interfaces. The adoption of EIS for obtaining analytical signals in biosensors based on aptamers is gaining popularity because of its advantageous characteristics for molecular recognition. Neuropeptide Y (NPY), the most abundant neuropeptide in the body, plays a crucial role with its stress-relieving properties. Quantitative measurement of NPY is imperative for understanding its role in these and other biological processes. Although aptamer-modified electrodes for NPY detection using EIS present a promising alternative, the correlation between the data obtained and the adsorption process on the electrodes is not fully understood. Various studies utilize the change in charge transfer resistance when employing an active redox label. In contrast, label-free measurement relies on changes in capacitance. To address these challenges, we focused on the interaction between aptamer-modified planar electrodes and their target, NPY. We proposed utilizing -ω*Zimag as the analytical signal, which facilitated the analysis of the adsorption process using an analogous Langmuir isotherm equation. This approach differs from implantable microelectrodes, which adhere to the Freundlich adsorption isotherm. Notably, our method obviates the need for a redox label and enables the detection of NPY at concentrations as low as 20 pg/mL. This methodology demonstrated exceptional selectivity, exhibiting a signal difference of over 20-to-1 against potential interfering molecules.
Keywords: Biosensing; Electrochemical impedance spectroscopy; Langmuir isotherm; Neuropeptide Y; Single layer adsorption.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no personal relationships or financial interests that could be perceived to have influenced this paper.
Figures
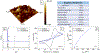
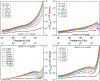


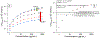
References
-
- Li R, Li X, Su L, Qi H, Yue X, Qi H, Label-free Electrochemical Aptasensor for the Determination of Serotonin, Electroanalysis 34 (2022) 1048–1053. 10.1002/elan.202100373. - DOI
-
- Ferapontova EE, Gothelf KV, Optimization of the electrochemical RNA-aptamer based biosensor for theophylline by using a methylene blue redox label, Electroanalysis 21 (2009) 1261–1266. 10.1002/elan.200804558. - DOI
-
- Weese-Myers ME, Ross AE, Electrochemical characterization of 17β-estradiol with fast-scan cyclic voltammetry, Electroanalysis 35 (2023). 10.1002/elan.202200560. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
