Gonadotropins Regulate the mRNA Expression of Gonadotropin-Releasing Hormone and Its Receptors in the Mouse Ovary and Uterus
- PMID: 38654976
- PMCID: PMC11034991
- DOI: 10.12717/DR.2024.28.1.1
Gonadotropins Regulate the mRNA Expression of Gonadotropin-Releasing Hormone and Its Receptors in the Mouse Ovary and Uterus
Abstract
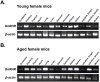
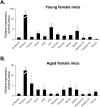
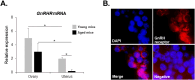
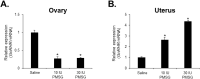
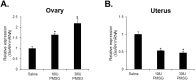
Gonadotropin-releasing hormone (GnRH), a critical hormone produced in the hypothalamus, is essential for regulating reproductive processes. It has also been demonstrated the presence of GnRH and its receptors (GnRHR) in ovarian and uterine tissues, but little was known about the regulation mechanism of their expression in these organs and ovarian aging. Therefore, the aim of this study was to investigate the expression of GnRHR in the ovary and uterus of mice, particularly after high-dose gonadotropin treatments and in relation to aging. Quantitative real-time-PCR (qRT-PCR) revealed that pituitary gland had the highest GnRHR expression in both young and aged mice. In addition, liver expression was higher in young mice, whereas thymus expression was higher in aged mice. GnRHR mRNA was present in the ovaries of both young and aged mice but nearly undetectable in the uterus of aged mice. We next examined the expression of GnRHR in the ovary and uterus in response to high-dose administration of pregnant mare serum gonadotropin (PMSG). After PMSG administration, GnRH mRNA levels were significantly decreased in the ovary but increased in the uterus. The expression of GnRH mRNA in these organs showed opposite trends to that of GnRHR expression. These results suggest the involvement of GnRH in age-related reproductive decline and the potential effects of high-dose gonadotropin treatments on reproductive organ function.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

