A robust heterogeneous chiral phosphoric acid enables multi decagram scale production of optically active N, S-ketals
- PMID: 38654978
- PMCID: PMC11033974
- DOI: 10.1039/d4gc00019f
A robust heterogeneous chiral phosphoric acid enables multi decagram scale production of optically active N, S-ketals
Abstract
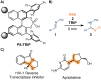
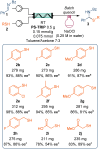
Asymmetric organocatalysis has been recognized as one of the "top 10 emerging technologies" in chemistry by IUPAC in 2019. Its potential to make chemical processes more sustainable is promising, but there are still challenges that need to be addressed. Developing new and reliable enantioselective processes for reproducing batch reactions on a large scale requires a combination of chemical and technical solutions. In this manuscript, we combine a robust immobilized chiral phosphoric acid with a new packed-bed reactor design. This combination allows scaling up of the enantioselective addition of thiols to imines from a few milligrams to a multi-decagram scale in a continuous flow process without physical or chemical degradation of the catalyst.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
-
Pioneering examples in asymmetric organocatalysis:
- Jen W. S. Wiener J. J. M. MacMillan D. W. C. J. Am. Chem. Soc. 2000;122:9874–9875. doi: 10.1021/ja005517p. - DOI
- Ahrendt K. A. Borths C. J. Macmillan D. W. C. J. Am. Chem. Soc. 2000;122:4243–4244. doi: 10.1021/ja000092s. - DOI
- List B. J. Am. Chem. Soc. 2000;122:9336–9337. doi: 10.1021/ja001923x. - DOI
- List B. Lerner R. A. Barbas III C. F. J. Am. Chem. Soc. 2000;122:2395–2396. doi: 10.1021/ja994280y. - DOI
- Akiyama T. Itoh J. Yokota K. Fuchibe K. Angew. Chem., Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. - DOI - PubMed
- Uraguchi D. Terada M. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. - DOI - PubMed
- Nakashima D. Yamamoto H. J. Am. Chem. Soc. 2006;128:9626–9627. doi: 10.1021/ja062508t. - DOI - PubMed
-
-
-
Recent reviews:
- Han B. He X. H. Liu Y. Q. He G. Peng C. Li J. L. Chem. Soc. Rev. 2021;50:1522–1586. doi: 10.1039/D0CS00196A. - DOI - PubMed
- Xiang S. H. Tan B. Nat. Commun. 2020;11:3786. doi: 10.1038/s41467-020-17580-z. - DOI - PMC - PubMed
- Carlone A. Bernardi L. Phys. Sci. Rev. 2019;4:1–21.
- del Corte X. Martínez de Marigorta E. Palacios F. Vicario J. Maestro A. Org. Chem. Front. 2022;9:6331–6399. doi: 10.1039/D2QO01209J. - DOI
-
-
- Gomollón-Bel F. Chem. Int. 2019;41:12–17.
LinkOut - more resources
Full Text Sources
Miscellaneous
