Long-term real-world data of ustekinumab in Crohn's disease: the Stockholm ustekinumab study
- PMID: 38655034
- PMCID: PMC11036920
- DOI: 10.1177/17562848241242700
Long-term real-world data of ustekinumab in Crohn's disease: the Stockholm ustekinumab study
Abstract
Background: Ustekinumab is used to treat inflammatory bowel disease mainly in patients failing anti-tumour necrosis factor (TNF)-agents.
Objectives: To provide real-world data in unselected patients with Crohn's disease (CD), treated with ustekinumab.
Design: Longitudinal retrospective study at four hospitals in Stockholm, Sweden.
Methods: Disease activity (Harvey-Bradshaw index and physician global assessment), laboratory parameters, endoscopic findings and drug persistence were assessed. Follow-up data were obtained in patients that stopped ustekinumab.
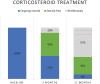
Results: In total, 322 patients (median age 38 years, 48% women) were included. All had luminal disease and 22% also fistulizing disease. A total of 271 (84%) had failed ⩾1 and 148 (46%) ⩾2 anti-TNF drugs; 34% failed vedolizumab. At inclusion, 93% had active disease; 28% were on oral corticosteroids and 18% on thiopurines. The median follow-up on treatment was 13.5 months; overall 67% were followed at least 24 months. By intention to treat analysis, response rate at 3 and 12 months was 43% and 42%, respectively. Among patients with ongoing ustekinumab, 19% were in steroid-free remission at 3 months and 64% at 12 months. The median faecal calprotectin level decreased from 460 µg/g at baseline to 156 µg/g at 3 months and was 182 µg/g at 12 months. C-reactive protein remained stable at 4 mg/L whereas serum albumin increased slightly. About 31% of patients were withdrawn during the first 12 months, mainly due to persisting disease activity 21%, adverse events 5%, bowel surgery 0.6% or malignancy 0.3%. The overall persistence on ustekinumab was 88%, 51%, 34% and 20% at 3, 12, 24 and 36 months, respectively. Within 12 months following withdrawal of ustekinumab in 121 patients, 64% had active disease most of the time, 68% needed another biologic and 24% underwent surgery.
Conclusion: Among difficult-to-treat patients with CD, ustekinumab was effective in the majority, with high drug persistence at 12 and 24 months in combination with a favourable safety profile.
Keywords: Crohn’s disease; long-term follow-up; real-world data; ustekinumab.
Plain language summary
Study of a new biologic treatment in patients with Crohn’s disease that have failed previous medications Why was the study done? New medical treatments become available in routine healthcare after rigorous testing on selected groups of patients in what is called clinical trials. The benefits and possible adverse events then need to be assessed in larger groups of unselected patients to gain a more generalizable knowledge of merits and shortfalls. Therefore studies in real-world settings are vital to complement the experience gained from clinical trials and they can provide robust data and reveal previously undetected safety issues. What did the researchers do? The research team studied the effect of a new injectable biological treatment, ustekinumab, in a large group of Swedish patients with an inflammatory bowel disease, Crohn’s disease, since the drug became available in routine health care. All patients had previously over long time periods tried several different treatments without obtaining stable symptom control. Information on the severity of disease, symptoms, laboratory tests, examinations performed, outcome and length of treatment and need for surgical interventions was retrieved from the medical records and further analyzed with statistical methods. What did the researchers find? Of all 322 patients 31% stopped the treatment within the first year, most often due to lack of stable symptom control or side effects. Less than one of 100 patients had to undergo surgery. More than half of all patients continued the treatment for at least one year and one-third for at least two years. Laboratory tests and endoscopic examinations used to assess ongoing inflammation improved. What do the findings mean? This study provides reliable information on the routine use of ustekinumab in patients with Crohn’s disease and persisting disease symptoms despite several previous treatments attempts. More than half of these difficult-to-treat patients had long-term benefit of this biologic and the drug was most often well tolerated.
© The Author(s), 2024.
Conflict of interest statement
FB, HS and AB performed part of the work during their internship in gastroenterology at Region Stockholm. FB: Speaker: Janssen, Research: Janssen. CH: speaker: Janssen, Vifor-Pharma, Takeda. OF and CW are employees of Janssen Cilag AB. SA: Scientific committee/Advisory board: Pharmacosmos, Janssen, Takeda, Consultant: Takeda, Janssen, Speaker: Galapagos, Janssen, Tillotts, Research: Janssen. The other authors have nothing to declare.
Figures





References
-
- Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimizing response to anti-TNF therapy in Crohn’s disease – algorithm for practical management. Aliment Pharmacol Ther 2016; 43: 30–51. - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al..; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. - PubMed
-
- Vermeire S, Loftus EV, Jr, Colombel JF, et al.. Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017; 11: 412–424. - PubMed
-
- Sandborn WJ, Gasink C, Gao LL, et al..; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012; 367: 1519–1528. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

