New insight into the biological activity of Salmo salar NK-lysin antimicrobial peptides
- PMID: 38655253
- PMCID: PMC11035819
- DOI: 10.3389/fimmu.2024.1191966
New insight into the biological activity of Salmo salar NK-lysin antimicrobial peptides
Abstract
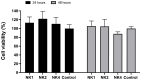
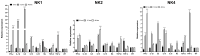
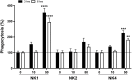
NK-lysin is a potent antimicrobial peptide (AMP) with antimicrobial activity against bacteria, fungi, viruses, and parasites. NK-lysin is a type of granulysin, a member of the saposin-like proteins family first isolated from a pig's small intestine. In previous work, for the first time, we identified four variants of nk-lysin from Atlantic salmon (Salmo salar) using EST sequences. In the present study, we reported and characterized two additional transcripts of NK-lysin from S. salar. Besides, we evaluated the tissue distribution of three NK-lysins from S. salar and assessed the antimicrobial, hemolytic, and immunomodulatory activities and signaling pathways of three NK-lysin-derived peptides. The synthetic peptides displayed antimicrobial activity against Piscirickettsia salmonis (LF-89) and Flavobacterium psychrophilum. These peptides induced the expression of immune genes related to innate and adaptive immune responses in vitro and in vivo. The immunomodulatory activity of the peptides involves the mitogen-activated protein kinases-mediated signaling pathway, including p38, extracellular signal-regulated kinase 1/2, and/or c-Jun N-terminal kinases. Besides, the peptides modulated the immune response induced by pathogen-associated molecular patterns (PAMPs). Our findings show that NK-lysin could be a highly effective immunostimulant or vaccine adjuvant for use in fish aquaculture.
Keywords: NK-lysin; Salmo salar; antimicrobial peptide; cytokines; immune response.
Copyright © 2024 Ortega, Carrera, Muñoz-Flores, Salazar, Villegas, Starck, Valenzuela, Agurto, Montesino, Astuya, Parra, Pérez, Santibáñez, Romero, Ruíz, Lamazares, Reyes, Sánchez, Toledo and Acosta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

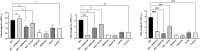
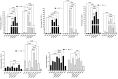
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

