Identification of a distinct cluster of GDF15high macrophages induced by in vitro differentiation exhibiting anti-inflammatory activities
- PMID: 38655264
- PMCID: PMC11036887
- DOI: 10.3389/fimmu.2024.1309739
Identification of a distinct cluster of GDF15high macrophages induced by in vitro differentiation exhibiting anti-inflammatory activities
Abstract
Introduction: Macrophage-mediated inflammatory response may have crucial roles in the pathogenesis of a variety of human diseases. Growth differentiation factor 15 (GDF15) is a cytokine of the transforming growth factor-β superfamily, with potential anti-inflammatory activities. Previous studies observed in human lungs some macrophages which expressed a high level of GDF15.
Methods: In the present study, we employed multiple techniques, including immunofluorescence, flow cytometry, and single-cell RNA sequencing, in order to further clarify the identity of such GDF15high macrophages.
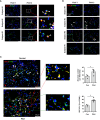
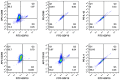
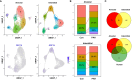
Results: We demonstrated that macrophages derived from human peripheral blood mononuclear cells and rat bone marrow mononuclear cells by in vitro differentiation with granulocyte-macrophage colony stimulating factor contained a minor population (~1%) of GDF15high cells. GDF15high macrophages did not exhibit a typical M1 or M2 phenotype, but had a unique molecular signature as revealed by single-cell RNA sequencing. Functionally, the in vitro derived GDF15high macrophages were associated with reduced responsiveness to pro-inflammatory activation; furthermore, these GDF15high macrophages could inhibit the pro-inflammatory functions of other macrophages via a paracrine mechanism. We further confirmed that GDF15 per se was a key mediator of the anti-inflammatory effects of GDF15high macrophage. Also, we provided evidence showing that GDF15high macrophages were present in other macrophage-residing human tissues in addition to the lungs. Further scRNA-seq analysis in rat lung macrophages confirmed the presence of a GDF15high sub-population. However, these data indicated that GDF15high macrophages in the body were not a uniform population based on their molecular signatures. More importantly, as compared to the in vitro derived GDF15high macrophage, whether the tissue resident GDF15high counterpart is also associated with anti-inflammatory functions remains to be determined. We cannot exclude the possibility that the in vitro priming/induction protocol used in our study has a determinant role in inducing the anti-inflammatory phenotype in the resulting GDF15high macrophage cells.
Conclusion: In summary, our results suggest that the GDF15high macrophage cells obtained by in vitro induction may represent a distinct cluster with intrinsic anti-inflammatory functions. The (patho)physiological importance of these cells in vivo warrants further investigation.
Keywords: GDF15; anti-inflammatory; cell population; growth differentiation factor 15; human; macrophage; single-cell RNA sequencing.
Copyright © 2024 Dai, Zhang, Zheng, Li, Ma, Gao, Zhang, Jiang and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

