Design, synthesis, biological and computational screening of novel pyridine-based thiadiazole derivatives as prospective anti-inflammatory agents
- PMID: 38655368
- PMCID: PMC11036016
- DOI: 10.1016/j.heliyon.2024.e29390
Design, synthesis, biological and computational screening of novel pyridine-based thiadiazole derivatives as prospective anti-inflammatory agents
Abstract
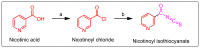
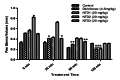
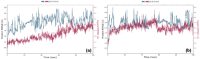
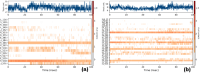
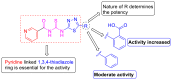
In this study, a novel series of pyridine-based thiadiazole derivatives (NTD1-NTD5) were synthesized as prospective anti-inflammatory agents by combining substituted carboxylic acid derivatives of 5-substituted-2-amino-1,3,4-thiadiazole with nicotinoyl isothiocyanate in the presence of acetone. The newly synthesized compounds were characterized by FTIR, 1H NMR, 13C NMR, and mass spectrometry. First, the compounds underwent rigorous in vivo testing for acute toxicity and anti-inflammatory activity and the results revealed that three compounds-NTD1, NTD2, and NTD3, displayed no acute toxicity and significant anti-inflammatory activity, surpassing the efficacy of the standard drug, diclofenac. Notably, NTD3, which featured benzoic acid substitution, emerged as the most potent anti-inflammatory agent among the screened compounds. To further validate these findings, an in silico docking study was carried out against COX-2 bound to diclofenac (PDB ID: 1pxx). The computational analysis demonstrated that NTD2, and NTD3, exhibited substantial binding affinity, with the lowest binding energies (-8.5 and -8.4, kcal/mol) compared to diclofenac (-8.4 kcal/mol). This alignment between in vivo and in silico data supported the robust anti-inflammatory potential of these derivatives. Moreover, molecular dynamics simulations were conducted, extending over 100 ns, to examine the dynamic interactions between the ligands and the target protein. The results solidified NTD3's position as a leading candidate, showing potent inhibitory activity through strong and sustained interactions, including stable hydrogen bond formations. This was further confirmed by RMSD values of 2-2.5 Å and 2-3Ǻ, reinforcing NTD3's potential as a useful anti-inflammatory agent. The drug likeness analysis of NTD3 through SwissADME indicated that most of the predicted parameters including Lipinski rule were within acceptable limits. While these findings are promising, further research is necessary to elucidate the precise relationships between the chemical structures and their activity, as well as to understand the mechanisms underlying their pharmacological effects. This study lays the foundation for the development of novel anti-inflammatory therapeutics, potentially offering improved efficacy and safety profiles.
Keywords: Acute toxicity; Anti-inflammatory activity; Cyclooxygenase-2; In silico studies; Pyridine derivatives; Thiadiazole derivatives.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











Similar articles
-
Synthesis, characterization, molecular docking and pharmacological evaluation of isoxazole derivatives as potent anti-inflammatory agents.Heliyon. 2024 Nov 15;10(22):e40300. doi: 10.1016/j.heliyon.2024.e40300. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39641075 Free PMC article.
-
Aryl/heteroaryl Substituted Celecoxib Derivatives as COX-2 Inhibitors: Synthesis, Anti-inflammatory Activity and Molecular Docking Studies.Med Chem. 2017;13(5):484-497. doi: 10.2174/1573406413666170221093740. Med Chem. 2017. PMID: 28228088
-
Design, Synthesis and Molecular Docking Studies of Novel Thiadiazole Analogues with Potential Antimicrobial and Antiinflammatory Activities.Antiinflamm Antiallergy Agents Med Chem. 2019;18(2):91-109. doi: 10.2174/1871520619666190307162442. Antiinflamm Antiallergy Agents Med Chem. 2019. PMID: 30848217
-
Synthesis, pharmacological screening and in silico studies of new class of Diclofenac analogues as a promising anti-inflammatory agents.Bioorg Med Chem. 2014 May 15;22(10):2855-66. doi: 10.1016/j.bmc.2014.03.043. Epub 2014 Apr 6. Bioorg Med Chem. 2014. PMID: 24751552
-
Synthesis of Novel Aryl (4-Aryl-1H-Pyrrol-3-yl) (Thiophen-2-yl) Methanone Derivatives: Molecular Modelling, In Silico ADMET, Anti-Inflammatory and Anti-Ulcer Activities.Antiinflamm Antiallergy Agents Med Chem. 2021;20(2):182-195. doi: 10.2174/1871523019999201116191622. Antiinflamm Antiallergy Agents Med Chem. 2021. PMID: 33200699
Cited by
-
Design, synthesis, in silico studies, and apoptotic antiproliferative activity of novel thiazole-2-acetamide derivatives as tubulin polymerization inhibitors.Front Chem. 2025 Apr 16;13:1565699. doi: 10.3389/fchem.2025.1565699. eCollection 2025. Front Chem. 2025. PMID: 40308265 Free PMC article.
-
Synthesis, characterization, molecular docking and pharmacological evaluation of isoxazole derivatives as potent anti-inflammatory agents.Heliyon. 2024 Nov 15;10(22):e40300. doi: 10.1016/j.heliyon.2024.e40300. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39641075 Free PMC article.
References
-
- Drożdżal S., Lechowicz K., Szostak B., Rosik J., Kotfis K., Machoy‐Mokrzyńska A., Białecka M., Ciechanowski K., Gawrońska‐Szklarz B. Kidney damage from nonsteroidal anti‐inflammatory drugs—myth or truth? Review of selected literature. Pharmacology Research & Perspectives. 2021;9(4) doi: 10.1002/prp2.817. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

