Skeletal rearrangement of 6,8-dioxabicyclo[3.2.1]octan-4-ols promoted by thionyl chloride or Appel conditions
- PMID: 38655557
- PMCID: PMC11035982
- DOI: 10.3762/bjoc.20.74
Skeletal rearrangement of 6,8-dioxabicyclo[3.2.1]octan-4-ols promoted by thionyl chloride or Appel conditions
Abstract
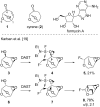
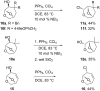
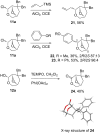
A skeletal rearrangement of a series of 6,8-dioxabicyclo[3.2.1]octan-4-ols has been developed using SOCl2 in the presence of pyridine. An oxygen migration from C5 to C4 was observed when the C4 alcohols were treated with SOCl2/pyridine, giving a 2-chloro-3,8-dioxabicyclo[3.2.1]octane ring-system via the chlorosulfite intermediate. Analogous allylic alcohols with endocyclic and exocyclic unsaturations underwent chlorination without rearrangement due to formation of allylic cations. The rearrangement was also demonstrated using Appel conditions, which gave similar results via the alkoxytriphenylphosphonium intermediate. Several reactions of the products were investigated to show the utility of the rearrangement.
Keywords: bicyclic ring; cyrene; levoglucosenone; rearrangement; thionyl chloride.
Copyright © 2024, Jevric et al.
Figures


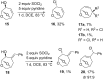

Similar articles
-
Acid-Catalyzed Redox Isomerizations of 6,8-Dioxabicyclo[3.2.1]octan-4-ols Provide Mechanistic Insights for Levoglucosenone Formation.ChemSusChem. 2025 May 5;18(9):e202402292. doi: 10.1002/cssc.202402292. Epub 2025 Jan 8. ChemSusChem. 2025. PMID: 39714994
-
Regioselective Synthesis of Acyclic cis-Enediynes via an Acid-Catalyzed Rearrangement of 1,2-Dialkynylallyl Alcohols. Syntheses, Computational Calculations, and Mechanism.J Org Chem. 1999 Jul 9;64(14):5062-5082. doi: 10.1021/jo982476v. J Org Chem. 1999. PMID: 34237883
-
Stereoretentive chlorination of cyclic alcohols catalyzed by titanium(IV) tetrachloride: evidence for a front side attack mechanism.J Org Chem. 2013 Mar 1;78(5):2118-27. doi: 10.1021/jo3023439. Epub 2013 Jan 23. J Org Chem. 2013. PMID: 23298402 Free PMC article.
-
Recent Advances in the Synthesis of β-Functionalized Ketones by Radical-Mediated 1,2-Rearrangement of Allylic Alcohols.Chemistry. 2018 Aug 1;24(43):10934-10947. doi: 10.1002/chem.201800004. Epub 2018 May 24. Chemistry. 2018. PMID: 29473267 Review.
-
[Cascade reactions of unsaturated xanthates and related reactions: computer-assisted molecular design and analysis of reaction mechanisms].Yakugaku Zasshi. 2005 Jun;125(6):469-89. doi: 10.1248/yakushi.125.469. Yakugaku Zasshi. 2005. PMID: 15930816 Review. Japanese.
Cited by
-
Controlled polymerization of levoglucosenone-derived enynes to give bio-based polymers with tunable degradation rates and high glass transition temperatures.Chem Sci. 2025 Apr 11;16(19):8435-8442. doi: 10.1039/d5sc00630a. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40225180 Free PMC article.
References
-
- Halpern Y, Riffer R, Broido A. J Org Chem. 1973;38:204–209. doi: 10.1021/jo00942a005. - DOI
-
- Kudo S, Huang X, Asano S, Hayashi J-i. Energy Fuels. 2021;35:9809–9824. doi: 10.1021/acs.energyfuels.1c01062. - DOI
-
- Klepp J, Dillon W, Lin Y, Feng P, Greatrex B W. Org Synth. 2020;97:38–53. doi: 10.15227/orgsyn.097.0038. - DOI
-
- Zhang J, White G B, Ryan M D, Hunt A J, Katz M J. ACS Sustainable Chem Eng. 2016;4:7186–7192. doi: 10.1021/acssuschemeng.6b02115. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
