Predictors of Success for Pulmonary Vein Isolation With Pulsed-field Ablation Using a Variable-loop Catheter With 3D Mapping Integration: Complete 12-month Outcomes From inspIRE
- PMID: 38655693
- PMCID: PMC11111320
- DOI: 10.1161/CIRCEP.123.012667
Predictors of Success for Pulmonary Vein Isolation With Pulsed-field Ablation Using a Variable-loop Catheter With 3D Mapping Integration: Complete 12-month Outcomes From inspIRE
Abstract
Background: We previously presented the safety and early efficacy of the inspIRE study (Study for Treatment of Paroxysmal Atrial Fibrillation [PAF] by Pulsed-field Ablation [PFA] System With Irreversible Electroporation [IRE]). With the study's conclusion, we report the outcomes of the full pivotal study cohort, with an additional analysis of predictors of success.
Methods: InspIRE was a prospective, multicenter, single-arm clinical trial of drug-refractory paroxysmal atrial fibrillation. Pulmonary vein isolation was performed with a variable-loop circular catheter integrated with a 3-dimensional mapping system. Follow-up with 24-hour Holter was at 3, 6, and 12 months, as well as remote rhythm monitoring: weekly from 3 to 5 months, monthly from 6 to 12 months, and for symptoms. The primary effectiveness end point (PEE) was acute pulmonary vein isolation plus freedom from any atrial arrhythmia at 12 months. Additional subanalyses report predictors of PEE success.
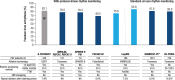
Results: The patient cohort included 186 patients: aged 59±10 years, female 30%, and CHA2DS2-VASc 1.3±1.2. The previously reported primary adverse event rate was 0%. One serious procedure-related adverse event, urinary retention, was reported. The PEE was achieved in 75.6% (95% CI, 69.5%-81.8%). The clinical success of freedom from symptomatic recurrence was 81.7% (95% CI, 76.1%-87.2%). Simulating a monitoring method used in standard real-world practice (without protocol-driven remote rhythm monitoring), this translates to a freedom from all and symptomatic recurrence of 85.8% (95% CI, 80.8%-90.9%) or 94.0% (95% CI, 90.6%-97.5%), respectively. Multivariate analyses revealed that left ventricular ejection fraction ≥60% (adjusted odds ratio, 0.30) and patients receiving ≥48 PFA applications (adjusted odds ratio, 0.28) were independent predictors of PEE success. Moreover, PEE success was 79.2% in patients who received ≥12 PFA applications per vein compared with 57.1% in patients receiving fewer PFA applications.
Conclusions: The inspIRE study confirms the safety and effectiveness of pulmonary vein isolation using the novel 3-dimensional mapping integrated circular loop catheter. An optimal number of PFA applications (≥48 total or ≥12 per vein) resulted in an improved 1-year success rate of ≈80%.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04524364.
Keywords: atrial fibrillation; catheters; electroporation; pulmonary veins.
Conflict of interest statement
Figures



References
-
- Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. . Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005 - PubMed
-
- Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE, 2nd. Primer on pulsed electrical field ablation: understanding the benefits and limitations. Circ Arrhythm Electrophysiol. 2021;14:e010086. doi: 10.1161/CIRCEP.121.010086 - PubMed
-
- Yavin H, Brem E, Zilberman I, Shapira-Daniels A, Datta K, Govari A, Anic A, Wazni O, Anter E. Circular multielectrode pulsed-field ablation catheter Lasso pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021;14:e009229. doi: 10.1161/CIRCEP.120.009229 - PMC - PubMed
-
- Reddy VY, Anter E, Rackauskas G, Peichl P, Koruth JS, Petru J, Funasako M, Minami K, Natale A, Jais P, et al. . Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol. 2020;13:e008718. doi: 10.1161/CIRCEP.120.008718 - PubMed
-
- Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, Cochet H, Minami K, Breskovic T, Sikiric I, et al. . Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

