Luminescent Pt(II) Complexes Using Unsymmetrical Bis(2-pyridylimino)isoindolate Analogues
- PMID: 38656154
- PMCID: PMC11080048
- DOI: 10.1021/acs.inorgchem.4c00558
Luminescent Pt(II) Complexes Using Unsymmetrical Bis(2-pyridylimino)isoindolate Analogues
Abstract
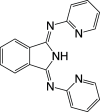
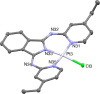
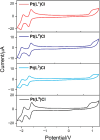
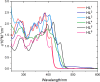
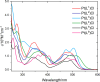
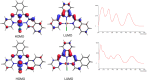
A series of ligands based upon a 1,3-diimino-isoindoline framework have been synthesized and investigated as pincer-type (N∧N∧N) chelates for Pt(II). The synthetic route allows different combinations of heterocyclic moieties (including pyridyl, thiazole, and isoquinoline) to yield new unsymmetrical ligands. Pt(L1-6)Cl complexes were obtained and characterized using a range of spectroscopic and analytical techniques: 1H and 13C NMR, IR, UV-vis and luminescence spectroscopies, elemental analyses, high-resolution mass spectrometry, electrochemistry, and one example via X-ray crystallography which showed a distorted square planar environment at Pt(II). Cyclic voltammetry on the complexes showed one irreversible oxidation between +0.75 and +1 V (attributed to Pt2+/3+ couple) and a number of ligand-based reductions; in four complexes, two fully reversible reductions were noted between -1.4 and -1.9 V. Photophysical studies showed that Pt(L1-6)Cl absorbs efficiently in the visible region through a combination of ligand-based bands and metal-to-ligand charge-transfer features at 400-550 nm, with assignments supported by DFT calculations. Excitation at 500 nm led to luminescence (studied in both solutions and solid state) in all cases with different combinations of the heterocyclic donors providing tuning of the emission wavelength around 550-678 nm.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sauer D. C.; Melen R. L.; Kruck M.; Gade L. H. Chromophores, Fluorophores and Robust Ancillary Ligands for Molecular Catalysts: 1,3-Bis(2-pyridylimino)-isoindolines. Eur. J. Inorg. Chem. 2014, 2014, 4715. 10.1002/ejic.201402595. - DOI
-
- Csonka R.; Speier G.; Kaizer J. Isoindoline-Derived Ligands and Applications. RSC Adv. 2015, 5, 18401–18419. 10.1039/C4RA15379K. - DOI
-
- Ho S. K. Y.; Lam F. Y. T.; de Aguirre A.; Maseras F.; White A. J. P.; Britovsek G. J. P. Photolytic Activation of Late-Transition-Metal–Carbon Bonds and Their Reactivity toward Oxygen. Organometallics 2021, 40, 4077–4091. 10.1021/acs.organomet.1c00487. - DOI
-
- Tseng K.-N. T.; Kampf J. W.; Szymczak N. K. Base-Free, Acceptorless, and Chemoselective Alcohol Dehydrogenation Catalyzed by an Amide-Derived NNN-Ruthenium(II) Hydride Complex. Organometallics 2013, 32, 2046–2049. 10.1021/om4000677. - DOI
- Gagne R. R.; Marks D. N. Ruthenium complexes of 1,3-bis(2-pyridylimino)isoindolines as alcohol oxidation catalysts. Inorg. Chem. 1984, 23, 65. 10.1021/ic00169a015. - DOI
-
- Muller A. L.; Wadepohl H.; Gade L. H. Bis(pyridylimino)isoindolato (BPI) Osmium Complexes: Structural Chemistry and Reactivity. Organometallics 2015, 34, 2810–2818. 10.1021/acs.organomet.5b00080. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

