Gut microbiota intervention alleviates pulmonary inflammation in broilers exposed to fine particulate matter from broiler house
- PMID: 38656183
- PMCID: PMC11107152
- DOI: 10.1128/aem.02174-23
Gut microbiota intervention alleviates pulmonary inflammation in broilers exposed to fine particulate matter from broiler house
Abstract
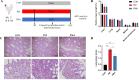
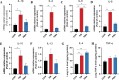
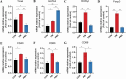
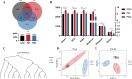
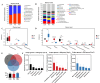
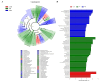
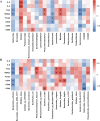
The gut microbiota of poultry is influenced by a variety of factors, including feed, drinking water, airborne dust, and footpads, among others. Gut microbiota can affect the immune reaction and inflammation in the lungs. To investigate the effect of gut microbiota variation on lung inflammation induced by PM2.5 (fine particulate matter) in broilers, 36 Arbor Acres (AA) broilers were randomly assigned to three groups: control group (CON), PM2.5 exposure group (PM), and PM2.5 exposure plus oral antibiotics group (PMA). We used non-absorbable antibiotics (ABX: neomycin and amikacin) to modify the microbiota composition in the PMA group. The intervention was conducted from the 18th to the 28th day of age. Broilers in the PM and PMA groups were exposed to PM by a systemic exposure method from 21 to 28 days old, and the concentration of PM2.5 was controlled at 2 mg/m3. At 28 days old, the lung injury score, relative mRNA expression of inflammatory factors, T-cell differentiation, and dendritic cell function were significantly increased in the PM group compared to the CON group, and those of the PMA group were significantly decreased compared to the PM group. There were significant differences in both α and β diversity of cecal microbiota among these three groups. Numerous bacterial genera showed significant differences in relative abundance among the three groups. In conclusion, gut microbiota could affect PM2.5-induced lung inflammation in broilers by adjusting the capacity of antigen-presenting cells to activate T-cell differentiation.
Importance: Gut microbes can influence the development of lung inflammation, and fine particulate matter collected from broiler houses can lead to lung inflammation in broilers. In this study, we explored the effect of gut microbes modified by intestinal non-absorbable antibiotics on particulate matter-induced lung inflammation. The results showed that modification in the composition of gut microbiota could alleviate lung inflammation by attenuating the ability of dendritic cells to stimulate T-cell differentiation, which provides a new way to protect lung health in poultry farms.
Keywords: T cell; dendritic cell; gut microbiota; particulate matter; pulmonary inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pulmonary microbiota intervention alleviates fine particulate matter-induced lung inflammation in broilers.J Anim Sci. 2023 Jan 3;101:skad207. doi: 10.1093/jas/skad207. J Anim Sci. 2023. PMID: 37341706 Free PMC article.
-
Effects of inhaled fine particulate matter on the lung injury as well as gut microbiota in broilers.Poult Sci. 2024 Apr;103(4):103426. doi: 10.1016/j.psj.2024.103426. Epub 2024 Jan 6. Poult Sci. 2024. PMID: 38335666 Free PMC article.
-
Inflammation-associated pulmonary microbiome and metabolome changes in broilers exposed to particulate matter in broiler houses.J Hazard Mater. 2022 Jan 5;421:126710. doi: 10.1016/j.jhazmat.2021.126710. Epub 2021 Jul 21. J Hazard Mater. 2022. PMID: 34332479
-
Alterations of lung and gut microbiota in sodium butyrate alleviating heat stress-induced lung injury of broilers.Poult Sci. 2025 Feb;104(2):104796. doi: 10.1016/j.psj.2025.104796. Epub 2025 Jan 9. Poult Sci. 2025. PMID: 39799858 Free PMC article.
-
Non-invasive biomarkers for monitoring intestinal health in broilers - A systematic review.Res Vet Sci. 2025 Jul;190:105669. doi: 10.1016/j.rvsc.2025.105669. Epub 2025 Apr 24. Res Vet Sci. 2025. PMID: 40306093
References
-
- Mignon-Grasteau S, Narcy A, Rideau N, Chantry-Darmon C, Boscher M-Y, Sellier N, Chabault M, Konsak-Ilievski B, Le Bihan-Duval E, Gabriel I. 2015. Impact of selection for digestive efficiency on microbiota composition in the chicken. PLOS ONE 10:e0135488. doi:10.1371/journal.pone.0135488 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

