How to use meropenem in pediatric patients undergoing CKRT? Integrated meropenem pharmacokinetic model for critically ill children
- PMID: 38656186
- PMCID: PMC11620509
- DOI: 10.1128/aac.01729-23
How to use meropenem in pediatric patients undergoing CKRT? Integrated meropenem pharmacokinetic model for critically ill children
Abstract
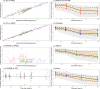
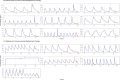
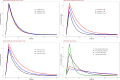
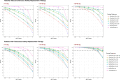
Standard dosing could fail to achieve adequate systemic concentrations in ICU children or may lead to toxicity in children with acute kidney injury. The population pharmacokinetic analysis was used to simultaneously analyze all available data (plasma, prefilter, postfilter, effluent, and urine concentrations) and provide the pharmacokinetic characteristics of meropenem. The probability of target fT > MIC attainment, avoiding toxic levels, during the entire dosing interval was estimated by simulation of different intermittent and continuous infusions in the studied population. A total of 16 critically ill children treated with meropenem were included, with 7 of them undergoing continuous kidney replacement therapy (CKRT). Only 33% of children without CKRT achieved 90% of the time when the free drug concentration exceeded the minimum inhibitory concentration (%fT > MIC) for an MIC of 2 mg/L. In dose simulations, only continuous infusions (60-120 mg/kg in a 24-h infusion) reached the objective in patients <30 kg. In patients undergoing CKRT, the currently used schedule (40 mg/kg/12 h from day 2 in a short infusion of 30 min) was clearly insufficient in patients <30 kg. Keeping the dose to 40 mg/kg q8h without applying renal adjustment and extended infusions (40 mg/kg in 3- or 4-h infusion every 12 h) was sufficient to reach 90% fT > MIC (>2 mg/L) in patients >10 kg. In patients <10 kg, only continuous infusions reached the objective. In patients >30 kg, 60 mg/kg in a 24-h infusion is sufficient and avoids toxicity. This population model could help with an individualized dosing approach that needs to be adopted in critically ill pediatric patients. Critically ill patients subjected to or not to CKRT may benefit from the administration of meropenem in an extended or continuous infusion.
Keywords: acute kidney injury; continuous kidney replacement therapy; continuous renal replacement therapy; critically ill children; dose individualization; meropenem; population pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Population Pharmacokinetics of Prolonged Infusion for Meropenem: Tailoring Dosing Recommendations for Chinese Critically Ill Patients on Continuous Renal Replacement Therapy with Consideration for Renal Function.Drug Des Devel Ther. 2025 Feb 17;19:1105-1117. doi: 10.2147/DDDT.S489603. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39991086 Free PMC article.
-
Population pharmacokinetics of piperacillin in critically ill children including those undergoing continuous kidney replacement therapy.Clin Microbiol Infect. 2022 Sep;28(9):1287.e9-1287.e15. doi: 10.1016/j.cmi.2022.03.031. Epub 2022 Apr 4. Clin Microbiol Infect. 2022. PMID: 35390523
-
Optimal Dosing of Meropenem in a Small Cohort of Critically Ill Children Receiving Continuous Renal Replacement Therapy.J Clin Pharmacol. 2021 Jun;61(6):744-754. doi: 10.1002/jcph.1798. Epub 2021 Jan 12. J Clin Pharmacol. 2021. PMID: 33314163 Free PMC article.
-
Pharmacokinetics of piperacillin and tazobactam in critically Ill patients treated with continuous kidney replacement therapy: A mini-review and population pharmacokinetic analysis.J Clin Pharm Ther. 2022 Aug;47(8):1091-1102. doi: 10.1111/jcpt.13657. Epub 2022 Mar 29. J Clin Pharm Ther. 2022. PMID: 35352374 Free PMC article. Review.
-
Optimal Meropenem Dosing Regimens in Patients Undergoing Continuous Renal Replacement Therapy: Systematic Review and Monte Carlo Simulations.Blood Purif. 2023;52(6):503-515. doi: 10.1159/000529694. Epub 2023 May 5. Blood Purif. 2023. PMID: 37231811
References
-
- European Medicines Agency . 2021. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. Available from: www.ema.europa.eu/en/use-pharmacokinetics-pharmacodynamics-development-a...
-
- Abdul-Aziz MH, Alffenaar J-W, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva J-A, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, Infection Section of European Society of Intensive Care Medicine (ESICM), Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT), Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC) . 2020. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med 46:1127–1153. doi:10.1007/s00134-020-06050-1 - DOI - PMC - PubMed
-
- Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, et al. . 2014. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi:10.1093/cid/ciu027 - DOI - PubMed
-
- Thy M, Urien S, Bouazza N, Foissac F, Gana I, Bille E, Béranger A, Toubiana J, Berthaud R, Lesage F, Renolleau S, Tréluyer J-M, Benaboud S, Oualha M. 2022. Meropenem population pharmacokinetics and dosing regimen optimization in critically ill children receiving continuous renal replacement therapy. Clin Pharmacokinet 61:1609–1621. doi:10.1007/s40262-022-01179-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

