Impact of Anti-angiogenic Drugs on Severity of COVID-19 in Patients with Non-Small Cell Lung Cancer
- PMID: 38656242
- PMCID: PMC11044805
- DOI: 10.1177/15330338241248573
Impact of Anti-angiogenic Drugs on Severity of COVID-19 in Patients with Non-Small Cell Lung Cancer
Abstract
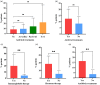
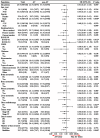
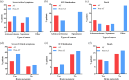
Introduction: The 2019 coronavirus disease (COVID-19) pandemic has reshaped oncology practice, but the impact of anti-angiogenic drugs on the severity of COVID-19 in patients with non-small cell lung cancer (NSCLC) remains unclear. Patients and Methods: We carried out a retrospective study involving 166 consecutive patients with NSCLC who were positive for COVID-19, aiming to determine the effects of anti-angiogenic drugs on disease severity, as defined by severe/critical symptoms, intensive care unit (ICU) admission/intubation, and mortality outcomes. Risk factors were identified using univariate and multivariate logistic regression models. Results: Of the participants, 73 had been administered anti-angiogenic drugs (termed the anti-angiogenic therapy (AT) group), while 93 had not (non-AT group). Comparative analyses showed no significant disparity in the rates of severe/critical symptoms (21.9% vs 35.5%, P = 0.057), ICU admission/intubation (6.8% vs 7.5%, P = 0.867), or death (11.0% vs 9.7%, P = 0.787) between these two groups. However, elevated risk factors for worse outcomes included age ≥ 60 (odds ratio (OR): 2.52, 95% confidence interval (CI): 1.07-5.92), Eastern Cooperative Oncology Group performance status of 2 or higher (OR: 21.29, 95% CI: 4.98-91.01), chronic obstructive pulmonary disease (OR: 7.25, 95% CI: 1.65-31.81), hypertension (OR: 2.98, 95% CI: 1.20-7.39), and use of immunoglobulin (OR: 5.26, 95% CI: 1.06-26.25). Conclusion: Our data suggests that the use of anti-angiogenic drugs may not exacerbate COVID-19 severity in NSCLC patients, indicating their potential safe application even during the pandemic period.
Keywords: COVID-19 severity; anti-angiogenic drugs; coronavirus disease 2019; non-small cell lung cancer; risk factors.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Risk of thromboembolism in patients with COVID-19 who are using hormonal contraception.Cochrane Database Syst Rev. 2023 Jan 9;1(1):CD014908. doi: 10.1002/14651858.CD014908.pub2. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2023 May 15;5:CD014908. doi: 10.1002/14651858.CD014908.pub3. PMID: 36622724 Free PMC article. Updated.
-
Inhaled corticosteroids for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Mar 9;3(3):CD015125. doi: 10.1002/14651858.CD015125. Cochrane Database Syst Rev. 2022. PMID: 35262185 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
-
- World Health Organization Data. WHO COVID-19 dashboard.[2023 November 22]. Available from:https://data.who.int/dashboards/covid19/cases?n=o.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

