Altered transcriptomic immune responses of maintenance hemodialysis patients to the COVID-19 mRNA vaccine
- PMID: 38656290
- PMCID: PMC11042800
- DOI: 10.7554/eLife.83641
Altered transcriptomic immune responses of maintenance hemodialysis patients to the COVID-19 mRNA vaccine
Abstract
Background: End-stage renal disease (ESRD) patients experience immune compromise characterized by complex alterations of both innate and adaptive immunity, and results in higher susceptibility to infection and lower response to vaccination. This immune compromise, coupled with greater risk of exposure to infectious disease at hemodialysis (HD) centers, underscores the need for examination of the immune response to the COVID-19 mRNA-based vaccines.
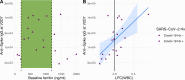
Methods: The immune response to the COVID-19 BNT162b2 mRNA vaccine was assessed in 20 HD patients and cohort-matched controls. RNA sequencing of peripheral blood mononuclear cells was performed longitudinally before and after each vaccination dose for a total of six time points per subject. Anti-spike antibody levels were quantified prior to the first vaccination dose (V1D0) and 7 d after the second dose (V2D7) using anti-spike IgG titers and antibody neutralization assays. Anti-spike IgG titers were additionally quantified 6 mo after initial vaccination. Clinical history and lab values in HD patients were obtained to identify predictors of vaccination response.
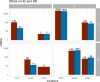
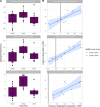
Results: Transcriptomic analyses demonstrated differing time courses of immune responses, with prolonged myeloid cell activity in HD at 1 wk after the first vaccination dose. HD also demonstrated decreased metabolic activity and decreased antigen presentation compared to controls after the second vaccination dose. Anti-spike IgG titers and neutralizing function were substantially elevated in both controls and HD at V2D7, with a small but significant reduction in titers in HD groups (p<0.05). Anti-spike IgG remained elevated above baseline at 6 mo in both subject groups. Anti-spike IgG titers at V2D7 were highly predictive of 6-month titer levels. Transcriptomic biomarkers after the second vaccination dose and clinical biomarkers including ferritin levels were found to be predictive of antibody development.
Conclusions: Overall, we demonstrate differing time courses of immune responses to the BTN162b2 mRNA COVID-19 vaccination in maintenance HD subjects comparable to healthy controls and identify transcriptomic and clinical predictors of anti-spike IgG titers in HD. Analyzing vaccination as an in vivo perturbation, our results warrant further characterization of the immune dysregulation of ESRD.
Funding: F30HD102093, F30HL151182, T32HL144909, R01HL138628. This research has been funded by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS) award UL1TR002003.
Keywords: COVID-19; RNA sequencing; binding antibodies; hemodialysis; human; infectious disease; medicine; microbiology; neutralizing antibodies; vaccine.
© 2024, Chang, Huang et al.
Conflict of interest statement
YC, KH, JL, CV, CA, MA, MG, CL, RE, YH, RC, NS, SW, SH, RG, BT, CS, MH, YC, JJ, WW, MR, PF, DP No competing interests declared, RN Received a grant from Janssen. The author has received consulting fees from Gilead and Viiv. The author has no other competing interests to declare
Figures


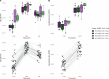
Update of
-
Immune response to the mRNA COVID-19 vaccine in hemodialysis patients: cohort study.medRxiv [Preprint]. 2023 Jan 19:2023.01.19.23284792. doi: 10.1101/2023.01.19.23284792. medRxiv. 2023. Update in: Elife. 2024 Apr 24;13:e83641. doi: 10.7554/eLife.83641. PMID: 36711520 Free PMC article. Updated. Preprint.
References
-
- Agur T, Ben-Dor N, Goldman S, Lichtenberg S, Herman-Edelstein M, Yahav D, Rozen-Zvi B, Zingerman B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients - a prospective cohort study. Nephrology, Dialysis, Transplantation. 2021;36:1347–1349. doi: 10.1093/ndt/gfab155. - DOI - PMC - PubMed
-
- Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF. A monoclonal antibody reactive with A 15-kDa cytoplasmic granule-associated protein defines A subpopulation of CD8+ T lymphocytes. Journal of Immunology. 1990;144:574–582. - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

