Chaperone-mediated MHC-I peptide exchange in antigen presentation
- PMID: 38656309
- PMCID: PMC11067752
- DOI: 10.1107/S2052252524002768
Chaperone-mediated MHC-I peptide exchange in antigen presentation
Abstract
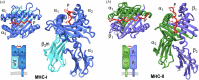
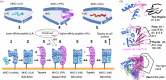
This work focuses on molecules that are encoded by the major histocompatibility complex (MHC) and that bind self-, foreign- or tumor-derived peptides and display these at the cell surface for recognition by receptors on T lymphocytes (T cell receptors, TCR) and natural killer (NK) cells. The past few decades have accumulated a vast knowledge base of the structures of MHC molecules and the complexes of MHC/TCR with specificity for many different peptides. In recent years, the structures of MHC-I molecules complexed with chaperones that assist in peptide loading have been revealed by X-ray crystallography and cryogenic electron microscopy. These structures have been further studied using mutagenesis, molecular dynamics and NMR approaches. This review summarizes the current structures and dynamic principles that govern peptide exchange as these relate to the process of antigen presentation.
Keywords: MHC; MHC-I/TAPBPR; MHC-I/tapasin; PLC; antigen presentation; chaperones; major histocompatibility complex; peptide exchange; structural immunology.
open access.
Figures







References
-
- Berman, H. M., Battistuz, T., Bhat, T. N., Bluhm, W. F., Bourne, P. E., Burkhardt, K., Feng, Z., Gilliland, G. L., Iype, L., Jain, S., Fagan, P., Marvin, J., Padilla, D., Ravichandran, V., Schneider, B., Thanki, N., Weissig, H., Westbrook, J. D. & Zardecki, C. (2002). Acta Cryst. D58, 899–907. - PubMed
-
- Blees, A., Januliene, D., Hofmann, T., Koller, N., Schmidt, C., Trowitzsch, S., Moeller, A. & Tampé, R. (2017). Nature, 551, 525–528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

