Stigmatization and Mental Health Impact of Chronic Pediatric Skin Disorders
- PMID: 38656377
- PMCID: PMC11044010
- DOI: 10.1001/jamadermatol.2024.0594
Stigmatization and Mental Health Impact of Chronic Pediatric Skin Disorders
Abstract
Importance: Chronic skin disorders in children frequently are visible and can cause stigmatization. However, the extent of stigmatization from chronic skin disease and association with mental health needs further study.
Objective: To examine the extent of stigma, dependence on disease visibility and severity, and association with mental health and quality of life (QOL) in chronic pediatric skin disease.
Design, setting, and participants: A cross-sectional, single-visit study was conducted at 32 pediatric dermatology centers in the US and Canada from November 14, 2018, to November 17, 2021. Participants included patients aged 8 to 17 years with chronic skin disease and 1 parent.
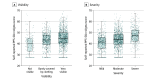
Main outcomes and measures: Using the Patient-Reported Outcomes Measurement Instrumentation System (PROMIS) Stigma-Skin, the extent of stigma with child-, caregiver-, and physician-assessed disease visibility (primary outcome) and severity was compared, as well as reduced QOL (assessed by Skindex-Teen), depression, anxiety, and poor peer relationships (PROMIS child and proxy tools) (secondary outcomes).
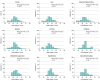
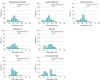
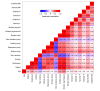
Results: The study included 1671 children (57.9% female; mean [SD] age, 13.7 [2.7] years). A total of 56.4% participants had self-reported high disease visibility and 50.5% had moderate disease severity. Stigma scores significantly differed by level of physician-assessed and child/proxy-assessed disease visibility and severity. Among children with chronic skin disorders, predominantly acne, atopic dermatitis, alopecia areata, and vitiligo, only 27.0% had T scores less than 40 (minimal or no stigma) and 43.8% had at least moderate stigma (T score ≥45) compared with children with a range of chronic diseases. Stigma scores correlated strongly with reduced QOL (Spearman ρ = 0.73), depression (ρ = 0.61), anxiety (ρ = 0.54), and poor peer relationships (ρ = -0.49). Overall, 29.4% of parents were aware of bullying of their child, which was strongly associated with stigma (Cohen d = -0.79, with children who were not bullied experiencing lower levels of stigma). Girls reported more stigma than boys (Cohen d = 0.26). Children with hyperhidrosis and hidradenitis suppurativa were most likely to have increased depression and anxiety.
Conclusions and relevance: The findings of this study suggest that physician assessment of disease severity and visibility is insufficient to evaluate the disease impact in the patient/caregiver. Identifying stigmatization, including bullying, and tracking improvement through medical and psychosocial interventions may be a key role for practitioners.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

