Transcription factor Nrf1 regulates proteotoxic stress-induced autophagy
- PMID: 38656405
- PMCID: PMC11040505
- DOI: 10.1083/jcb.202306150
Transcription factor Nrf1 regulates proteotoxic stress-induced autophagy
Abstract
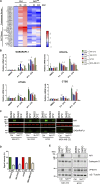
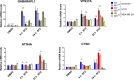
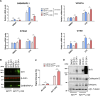
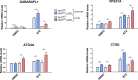
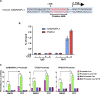
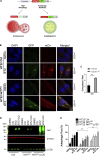
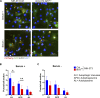
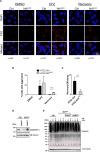
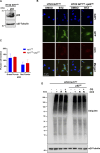
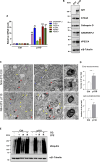
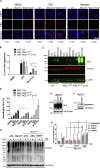
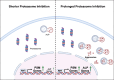
Cells exposed to proteotoxic stress invoke adaptive responses aimed at restoring proteostasis. Our previous studies have established a firm role for the transcription factor Nuclear factor-erythroid derived-2-related factor-1 (Nrf1) in responding to proteotoxic stress elicited by inhibition of cellular proteasome. Following proteasome inhibition, Nrf1 mediates new proteasome synthesis, thus enabling the cells to mitigate the proteotoxic stress. Here, we report that under similar circumstances, multiple components of the autophagy-lysosomal pathway (ALP) were transcriptionally upregulated in an Nrf1-dependent fashion, thus providing the cells with an additional route to cope with proteasome insufficiency. In response to proteasome inhibitors, Nrf1-deficient cells displayed profound defects in invoking autophagy and clearance of aggresomes. This phenomenon was also recapitulated in NGLY1 knockout cells, where Nrf1 is known to be non-functional. Conversely, overexpression of Nrf1 induced ALP genes and endowed the cells with an increased capacity to clear aggresomes. Overall, our results significantly expand the role of Nrf1 in shaping the cellular response to proteotoxic stress.
© 2024 Ward et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

