Neurodevelopmental Outcomes After Late Preterm Antenatal Corticosteroids: The ALPS Follow-Up Study
- PMID: 38656759
- PMCID: PMC11044009
- DOI: 10.1001/jama.2024.4303
Neurodevelopmental Outcomes After Late Preterm Antenatal Corticosteroids: The ALPS Follow-Up Study
Erratum in
-
Data Errors.JAMA. 2025 Apr 22;333(16):1460. doi: 10.1001/jama.2025.4205. JAMA. 2025. PMID: 40146167 Free PMC article. No abstract available.
Abstract
Importance: The Antenatal Late Preterm Steroids (ALPS) trial changed clinical practice in the United States by finding that antenatal betamethasone at 34 to 36 weeks decreased short-term neonatal respiratory morbidity. However, the trial also found increased risk of neonatal hypoglycemia after betamethasone. This follow-up study focused on long-term neurodevelopmental outcomes after late preterm steroids.
Objective: To evaluate whether administration of late preterm (34-36 completed weeks) corticosteroids affected childhood neurodevelopmental outcomes.
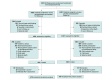
Design, setting, and participants: Prospective follow-up study of children aged 6 years or older whose birthing parent had enrolled in the multicenter randomized clinical trial, conducted at 13 centers that participated in the Maternal-Fetal Medicine Units (MFMU) Network cycle from 2011-2016. Follow-up was from 2017-2022.
Exposure: Twelve milligrams of intramuscular betamethasone administered twice 24 hours apart.
Main outcome and measures: The primary outcome of this follow-up study was a General Conceptual Ability score less than 85 (-1 SD) on the Differential Ability Scales, 2nd Edition (DAS-II). Secondary outcomes included the Gross Motor Function Classification System level and Social Responsiveness Scale and Child Behavior Checklist scores. Multivariable analyses adjusted for prespecified variables known to be associated with the primary outcome. Sensitivity analyses used inverse probability weighting and also modeled the outcome for those lost to follow-up.
Results: Of 2831 children, 1026 enrolled and 949 (479 betamethasone, 470 placebo) completed the DAS-II at a median age of 7 years (IQR, 6.6-7.6 years). Maternal, neonatal, and childhood characteristics were similar between groups except that neonatal hypoglycemia was more common in the betamethasone group. There were no differences in the primary outcome, a general conceptual ability score less than 85, which occurred in 82 (17.1%) of the betamethasone vs 87 (18.5%) of the placebo group (adjusted relative risk, 0.94; 95% CI, 0.73-1.22). No differences in secondary outcomes were observed. Sensitivity analyses using inverse probability weighting or assigning outcomes to children lost to follow-up also found no differences between groups.
Conclusion and relevance: In this follow-up study of a randomized clinical trial, administration of antenatal corticosteroids to persons at risk of late preterm delivery, originally shown to improve short-term neonatal respiratory outcomes but with an increased rate of hypoglycemia, was not associated with adverse childhood neurodevelopmental outcomes at age 6 years or older.
Conflict of interest statement
Figures
Comment in
-
Late Preterm Corticosteroids Exposure and Neurodevelopmental Outcomes.JAMA. 2024 May 21;331(19):1626-1627. doi: 10.1001/jama.2024.2228. JAMA. 2024. PMID: 38656755 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- UG1 HD040485/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- UG1 HD068258/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- R01 HL098354/HL/NHLBI NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- UG1 HD068282/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD068268/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

