Pseudomonas aeruginosa Lipid A Structural Variants Induce Altered Immune Responses
- PMID: 38656811
- PMCID: PMC11299085
- DOI: 10.1165/rcmb.2024-0059OC
Pseudomonas aeruginosa Lipid A Structural Variants Induce Altered Immune Responses
Abstract
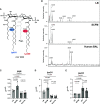
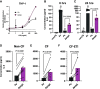
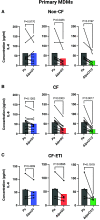
Pseudomonas aeruginosa causes chronic lung infection in cystic fibrosis (CF), resulting in structural lung damage and progressive pulmonary decline. P. aeruginosa in the CF lung undergoes numerous changes, adapting to host-specific airway pressures while establishing chronic infection. P. aeruginosa undergoes lipid A structural modification during CF chronic infection that is not seen in any other disease state. Lipid A, the membrane anchor of LPS (i.e., endotoxin), comprises the majority of the outer membrane of Gram-negative bacteria and is a potent Toll-like receptor 4 (TLR4) agonist. The structure of P. aeruginosa lipid A is intimately linked with its recognition by TLR4 and subsequent immune response. Prior work has identified P. aeruginosa strains with altered lipid A structures that arise during chronic CF lung infection; however, the impact of the P. aeruginosa lipid A structure on airway disease has not been investigated. Here, we show that P. aeruginosa lipid A lacks PagL-mediated deacylation during human airway infection using a direct-from-sample mass spectrometry approach on human BAL fluid. This structure triggers increased proinflammatory cytokine production by primary human macrophages. Furthermore, alterations in lipid A 2-hydroxylation impact cytokine response in a site-specific manner, independent of CF transmembrane conductance regulator function. It is interesting that there is a CF-specific reduction in IL-8 secretion within the epithelial-cell compartment that only occurs in CF bronchial epithelial cells when infected with CF-adapted P. aeruginosa that lacks PagL-mediated lipid A deacylation. Taken together, we show that P. aeruginosa alters its lipid A structure during acute lung infection and that this lipid A structure induces stronger signaling through TLR4.
Keywords: LPS; Pseudomonas; TLR4; airway adaptation; cystic fibrosis.
Figures



Similar articles
-
P. aeruginosa tRNA-fMet halves secreted in outer membrane vesicles suppress lung inflammation in cystic fibrosis.Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L574-L588. doi: 10.1152/ajplung.00018.2024. Epub 2024 Mar 5. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38440830 Free PMC article.
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2013 Dec 23;(12):CD009530. doi: 10.1002/14651858.CD009530.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Jan 21;(1):CD009530. doi: 10.1002/14651858.CD009530.pub3. PMID: 24363033 Updated.
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2024 May 3;5(5):CD009530. doi: 10.1002/14651858.CD009530.pub5. Cochrane Database Syst Rev. 2024. PMID: 38700027 Free PMC article.
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2016 Jan 21;(1):CD009530. doi: 10.1002/14651858.CD009530.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2018 Sep 17;9:CD009530. doi: 10.1002/14651858.CD009530.pub4. PMID: 26797965 Updated.
Cited by
-
Biotherapeutic potential of different fractions of cell-free supernatants from Lacticaseibacillus rhamnosus against Pseudomonas aeruginosa.Front Cell Infect Microbiol. 2025 Jun 30;15:1608897. doi: 10.3389/fcimb.2025.1608897. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40661974 Free PMC article.
-
Magnesium depletion unleashes two unusual modes of colistin resistance with different fitness costs.bioRxiv [Preprint]. 2025 Jun 25:2024.10.15.618514. doi: 10.1101/2024.10.15.618514. bioRxiv. 2025. PMID: 39464160 Free PMC article. Preprint.
-
Trimming the fat: a brief review of lipids at the host-pathogen interface.Infect Immun. 2025 Jul 8;93(7):e0050624. doi: 10.1128/iai.00506-24. Epub 2025 Jun 13. Infect Immun. 2025. PMID: 40512027 Free PMC article. Review.
-
Exploiting gasdermin-mediated pyroptosis for enhanced antimicrobial activity of phage endolysin against Pseudomonas aeruginosa.mSystems. 2025 Jan 21;10(1):e0110624. doi: 10.1128/msystems.01106-24. Epub 2024 Dec 23. mSystems. 2025. PMID: 39714210 Free PMC article.
-
Advances in Helicobacter pylori lipopolysaccharide structure and function.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf034. doi: 10.1093/femsre/fuaf034. FEMS Microbiol Rev. 2025. PMID: 40720770 Free PMC article. Review.
References
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med . 1996;154:1229–1256. - PubMed
-
- Dorwart M, Thibodeau P, Thomas P. Cystic fibrosis: recent structural insights. J Cyst Fibros . 2004;3:91–94. - PubMed
-
- Bergeron C, Cantin AM. Cystic fibrosis: pathophysiology of lung disease. Semin Respir Crit Care Med . 2019;40:715–726. - PubMed
-
- Ramsay KA, Stockwell RE, Bell SC, Kidd TJ. Infection in cystic fibrosis: impact of the environment and climate. Expert Rev Respir Med . 2016;10:505–519. - PubMed
-
- Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev . 2002;3:128–134. - PubMed
MeSH terms
Substances
Grants and funding
- T32HL007586/HL/NHLBI NIH HHS/United States
- T32 AI162579/AI/NIAID NIH HHS/United States
- R01Al147314/National Institute of Allergy and Infectious Diseases/United States
- T32 HL007586/HL/NHLBI NIH HHS/United States
- U19 AI110820/AI/NIAID NIH HHS/United States
- R01 AI147314/AI/NIAID NIH HHS/United States
- HOFSTA23H0/Cystic Fibrosis Foundation/United States
- T32AI162579/National Institute of Allergy and Infectious Diseases/United States
- ERNST23G0/Cystic Fibrosis Foundation/United States
- R01Al104895/National Institute of Allergy and Infectious Diseases/United States
LinkOut - more resources
Full Text Sources
Medical

