Long-term hematopoietic stem cells trigger quiescence in Leishmania parasites
- PMID: 38656959
- PMCID: PMC11073788
- DOI: 10.1371/journal.ppat.1012181
Long-term hematopoietic stem cells trigger quiescence in Leishmania parasites
Abstract
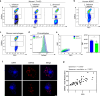
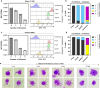
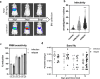
Addressing the challenges of quiescence and post-treatment relapse is of utmost importance in the microbiology field. This study shows that Leishmania infantum and L. donovani parasites rapidly enter into quiescence after an estimated 2-3 divisions in both human and mouse bone marrow stem cells. Interestingly, this behavior is not observed in macrophages, which are the primary host cells of the Leishmania parasite. Transcriptional comparison of the quiescent and non-quiescent metabolic states confirmed the overall decrease of gene expression as a hallmark of quiescence. Quiescent amastigotes display a reduced size and signs of a rapid evolutionary adaptation response with genetic alterations. Our study provides further evidence that this quiescent state significantly enhances resistance to treatment. Moreover, transitioning through quiescence is highly compatible with sand fly transmission and increases the potential of parasites to infect cells. Collectively, this work identified stem cells in the bone marrow as a niche where Leishmania quiescence occurs, with important implications for antiparasitic treatment and acquisition of virulence traits.
Copyright: © 2024 Dirkx et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

