Excited State Dynamics in Unidirectional Photochemical Molecular Motors
- PMID: 38656968
- PMCID: PMC11082934
- DOI: 10.1021/jacs.4c01019
Excited State Dynamics in Unidirectional Photochemical Molecular Motors
Abstract
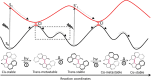
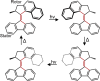
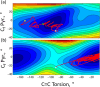
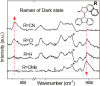
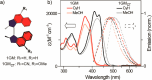
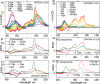
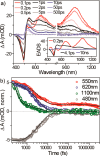
Unidirectional photochemically driven molecular motors (PMMs) convert the energy of absorbed light into continuous rotational motion. As such they are key components in the design of molecular machines. The prototypical and most widely employed class of PMMs is the overcrowded alkenes, where rotational motion is driven by successive photoisomerization and thermal helix inversion steps. The efficiency of such PMMs depends upon the speed of rotation, determined by the rate of ground state thermal helix inversion, and the quantum yield of photoisomerization, which is dependent on the excited state energy landscape. The former has been optimized by synthetic modification across three generations of overcrowded alkene PMMs. These improvements have often been at the expense of photoisomerization yield, where there remains room for improvement. In this perspective we review the application of ultrafast spectroscopy to characterize the excited state dynamics in PMMs. These measurements lead to a general mechanism for all generations of PMMs, involving subpicosecond decay of a Franck-Condon excited state to populate a dark excited state which decays within picoseconds via conical intersections with the electronic ground state. The model is discussed in the context of excited state dynamics calculations. Studies of PMM photochemical dynamics as a function of solvent suggest exploitation of intramolecular charge transfer and solvent polarity as a route to controlling photoisomerization yield. A test of these ideas for a first generation motor reveals a high degree of solvent control over isomerization yield. These results suggest a pathway to fine control over the performance of future PMMs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

