SARS-CoV-2 RNA and Nucleocapsid Antigen Are Blood Biomarkers Associated With Severe Disease Outcomes That Improve in Response to Remdesivir
- PMID: 38657001
- PMCID: PMC11420797
- DOI: 10.1093/infdis/jiae198
SARS-CoV-2 RNA and Nucleocapsid Antigen Are Blood Biomarkers Associated With Severe Disease Outcomes That Improve in Response to Remdesivir
Abstract
Background: Although antivirals remain important for the treatment COVID-19, methods to assess treatment efficacy are lacking. Here, we investigated the impact of remdesivir on viral dynamics and their contribution to understanding antiviral efficacy in the multicenter Adaptive COVID-19 Treatment Trial 1, which randomized patients to remdesivir or placebo.
Methods: Longitudinal specimens collected during hospitalization from a substudy of 642 patients with COVID-19 were measured for viral RNA (upper respiratory tract and plasma), viral nucleocapsid antigen (serum), and host immunologic markers. Associations with clinical outcomes and response to therapy were assessed.
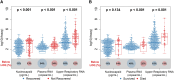
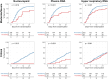
Results: Higher baseline plasma viral loads were associated with poorer clinical outcomes, and decreases in viral RNA and antigen in blood but not the upper respiratory tract correlated with enhanced benefit from remdesivir. The treatment effect of remdesivir was most pronounced in patients with elevated baseline nucleocapsid antigen levels: the recovery rate ratio was 1.95 (95% CI, 1.40-2.71) for levels >245 pg/mL vs 1.04 (95% CI, .76-1.42) for levels <245 pg/mL. Remdesivir also accelerated the rate of viral RNA and antigen clearance in blood, and patients whose blood levels decreased were more likely to recover and survive.
Conclusions: Reductions in SARS-CoV-2 RNA and antigen levels in blood correlated with clinical benefit from antiviral therapy.
Clinical trial registration: NCT04280705 (ClinicalTrials.gov).
Keywords: COVID-19; SARS-CoV-2; antiviral efficacy; clinical trial; remdesivir.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2024.
Conflict of interest statement
Potential conflicts of interest . A. L. G. reports contract testing to his institution from Abbott, Cepheid, Novavax, Pfizer, Janssen, and Hologic and research support to his institution from Gilead Sciences, Inc, and Merck outside of the described work. D. P. is an employee and shareholder of Gilead Sciences, Inc. K. J. is a shareholder of and was employed by Gilead Sciences, Inc, at the time of manuscript development. C. A. B. has received grants/contracts to her institution from Gilead Sciences, Inc, and consultant fees from NDA Partners, LLC, related to drug development. C. A. B. served as the president of the CROI Foundation Board of Directors, vice president of the Zimbabwe AIDS Treatment Assistance Project Board of Directors, and secretary/treasurer of the IAS-USA Board of Directors (all nonprofit organizations). C. A. B. recently served as deputy editor for Clinical Infectious Diseases for the Infectious Diseases Society of America. N. J. reports salary support by Sagent Pharmaceuticals to his institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K08 AI143923/AI/NIAID NIH HHS/United States
- U54 AG062334/AG/NIA NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- HHSN261200800001E 75N910D00024/CA/NCI NIH HHS/United States
- R38 AI174306/AI/NIAID NIH HHS/United States
- London International Coordinating Centre
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- National Institute of Allergy and Infectious Diseases
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- Seoul National University Hospital
- I01 CX002688/CX/CSRD VA/United States
- MRC_UU_12023/23/MRC_/Medical Research Council/United Kingdom
- NH/NIH HHS/United States

