Reversible strain-promoted DNA polymerization
- PMID: 38657068
- PMCID: PMC11042731
- DOI: 10.1126/sciadv.ado8020
Reversible strain-promoted DNA polymerization
Abstract
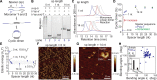
Molecular strain can be introduced to influence the outcome of chemical reactions. Once a thermodynamic product is formed, however, reversing the course of a strain-promoted reaction is challenging. Here, a reversible, strain-promoted polymerization in cyclic DNA is reported. The use of nonhybridizing, single-stranded spacers as short as a single nucleotide in length can promote DNA cyclization. Molecular strain is generated by duplexing the spacers, leading to ring opening and subsequent polymerization. Then, removal of the strain-generating duplexers triggers depolymerization and cyclic dimer recovery via enthalpy-driven cyclization and entropy-mediated ring contraction. This reversibility is retained even when a protein is conjugated to the DNA strands, and the architecture of the protein assemblies can be modulated between bivalent and polyvalent states. This work underscores the utility of using DNA not only as a programmable ligand for assembly but also as a route to access restorable bonds, thus providing a molecular basis for DNA-based materials with shape-memory, self-healing, and stimuli-responsive properties.
Figures




References
-
- Bodwell G. J., Bridson J. N., Houghton T. J., Kennedy J. W. J., Mannion M. R., 1,7-Dioxa[7](2,7)pyrenophane: The pyrene moiety is more bent than that of C70. Chem. A Eur. J. 5, 1823–1827 (1999).
-
- Zhang X., Mackinnon M. R., Bodwell G. J., Ito S., Synthesis of a π-extended azacorannulenophane enabled by strain-induced 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 61, e202116585 (2022). - PubMed
-
- Zholdassov Y. S., Yuan L., Garcia S. R., Kwok R. W., Boscoboinik A., Valles D. J., Marianski M., Martini A., Carpick R. W., Braunschweig A. B., Acceleration of Diels-Alder reactions by mechanical distortion. Science 380, 1053–1058 (2023). - PubMed
-
- Jiang L., Peng Z., Liang Y., Tang Z., Liang K., Liu J., Liu Z., Strain-driven formal [1,3]-aryl shift within molecular bows. Angew. Chem. Int. Ed. 62, e202312238 (2023). - PubMed
-
- Agard N. J., Prescher J. A., Bertozzi C. R., A strain-promoted [3 + 2] azide−alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

