NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer
- PMID: 38657187
- PMCID: PMC11227306
- DOI: 10.1200/JCO.23.01983
NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer
Abstract
Purpose: The anti-NECTIN4 antibody-drug conjugate enfortumab vedotin (EV) is approved for patients with metastatic urothelial cancer (mUC). However, durable benefit is only achieved in a small, yet uncharacterized patient subset. NECTIN4 is located on chromosome 1q23.3, and 1q23.3 gains represent frequent copy number variations (CNVs) in urothelial cancer. Here, we aimed to evaluate NECTIN4 amplifications as a genomic biomarker to predict EV response in patients with mUC.
Materials and methods: We established a NECTIN4-specific fluorescence in situ hybridization (FISH) assay to assess the predictive value of NECTIN4 CNVs in a multicenter EV-treated mUC patient cohort (mUC-EV, n = 108). CNVs were correlated with membranous NECTIN4 protein expression, EV treatment responses, and outcomes. We also assessed the prognostic value of NECTIN4 CNVs measured in metastatic biopsies of non-EV-treated mUC (mUC-non-EV, n = 103). Furthermore, we queried The Cancer Genome Atlas (TCGA) data sets (10,712 patients across 32 cancer types) for NECTIN4 CNVs.
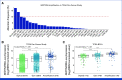
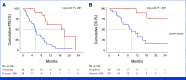
Results: NECTIN4 amplifications are frequent genomic events in muscle-invasive bladder cancer (TCGA bladder cancer data set: approximately 17%) and mUC (approximately 26% in our mUC cohorts). In mUC-EV, NECTIN4 amplification represents a stable genomic alteration during metastatic progression and associates with enhanced membranous NECTIN4 protein expression. Ninety-six percent (27 of 28) of patients with NECTIN4 amplifications demonstrated objective responses to EV compared with 32% (24 of 74) in the nonamplified subgroup (P < .001). In multivariable Cox analysis adjusted for age, sex, and Bellmunt risk factors, NECTIN4 amplifications led to a 92% risk reduction for death (hazard ratio, 0.08 [95% CI, 0.02 to 0.34]; P < .001). In the mUC-non-EV, NECTIN4 amplifications were not associated with outcomes. TCGA Pan-Cancer analysis demonstrated that NECTIN4 amplifications occur frequently in other cancers, for example, in 5%-10% of breast and lung cancers.
Conclusion: NECTIN4 amplifications are genomic predictors of EV responses and long-term survival in patients with mUC.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures




References
-
- Yu EY, Petrylak DP, O’Donnell PH, et al. : Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol 22:872-882, 2021 - PubMed
-
- Powles TB, Perez Valderrama B, Gupta S, et al. : LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann Oncol 34:S1340, 2023
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

