Correlation of immune fitness with response to teclistamab in relapsed/refractory multiple myeloma in the MajesTEC-1 study
- PMID: 38657201
- PMCID: PMC11347796
- DOI: 10.1182/blood.2023022823
Correlation of immune fitness with response to teclistamab in relapsed/refractory multiple myeloma in the MajesTEC-1 study
Abstract
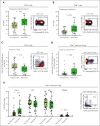
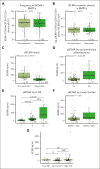
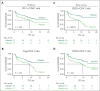
Teclistamab, an off-the-shelf B-cell maturation antigen (BCMA) × CD3 bispecific antibody that mediates T-cell activation and subsequent lysis of BCMA-expressing myeloma cells, is approved for the treatment of patients with relapsed/refractory multiple myeloma (R/RMM). As a T-cell redirection therapy, clinical outcomes with teclistamab may be influenced by patient immune fitness and tumor antigen expression. We correlated tumor characteristics and baseline immune profiles with clinical response and disease burden in patients with R/RMM from the pivotal phase 1/2 MajesTEC-1 study, focusing on patients treated with 1.5 mg/kg of teclistamab (N = 165). Peripheral blood samples were collected at screening, and bone marrow samples were collected at screening and cycle 3. Better clinical outcomes to teclistamab correlated with higher baseline total T-cell counts in the periphery. In addition, responders (partial response or better) had a lower proportion of immunosuppressive regulatory T cells (Tregs), T cells expressing coinhibitory receptors (CD38, PD-1, and PD-1/TIM-3), and soluble BCMA and a T-cell profile suggestive of a more cytolytic potential, compared with nonresponders. Neither frequency of baseline bone marrow BCMA expression nor BCMA-receptor density was associated with clinical response to teclistamab. Improved progression-free survival was observed in patients with a lower frequency of T cells expressing exhaustion markers and immunosuppressive Tregs. Overall, response to teclistamab was associated with baseline immune fitness; nonresponders had immune profiles suggestive of immune suppression and T-cell dysfunction. These findings illustrate the importance of the contribution of the immune landscape to T-cell redirection therapy response. This trial was registered at www.ClinicalTrials.gov as #NCT03145181/NCT04557098.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.C.-S., D.V., S. Skerget, T.P., S. Stein, R.B., O.S.L., K.N., C.D., J.P., A.B., T.S., C.U., R.K., L.P., D.T., and S.X.W.L. are employees of Janssen and may have stock/other ownership interests in Janssen. L.S.W., J.G., and S.G. were employees of Janssen at the time the work was carried out and may have stock/other ownership interests in Janssen. R.I.V. was an employee of Janssen at the time the work was carried out and may have stock/other ownership interests in Janssen. P.M. has served in consulting/advisory roles and received honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, and Sanofi. S.Z.U. has served in a consulting or advisory role for AbbVie, Amgen, Bristol Myers Squibb/Celgene, Celgene, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Merck, and Takeda; and has received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, and Skyline Diagnostics. N.J.B. has served in consulting/advisory roles for Amgen, Celgene, Janssen, Karyopharm Therapeutics, Pfizer, Sanofi, and Takeda; received honoraria from AbbVie, Amgen, Celgene, Genentech/Roche, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Sanofi, and Takeda; and has received research funding from Celgene and Janssen. N.W.C.J.v.d.D. has served in consulting/advisory roles for AbbVie, Adaptive Biotechnologies, Amgen, Bayer, Bristol Myers Squibb, Celgene, Janssen, Novartis, Pfizer, Merck, Roche, Servier, and Takeda; and has received research funding from Amgen, BMS, Celgene, Cellectis, Janssen, and Novartis.
The current affiliation for R.I.V. is AbbVie Inc, North Chicago, IL.
Figures




Comment in
-
Immunotherapy: the teclistamab fitness test.Blood. 2024 Aug 8;144(6):591-592. doi: 10.1182/blood.2024025046. Blood. 2024. PMID: 39115825 No abstract available.
References
-
- Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464–477. - PubMed
-
- Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152. - PubMed
-
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–3901. - PubMed
-
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

