SORL1 is a receptor for tau that promotes tau seeding
- PMID: 38657864
- PMCID: PMC11145553
- DOI: 10.1016/j.jbc.2024.107313
SORL1 is a receptor for tau that promotes tau seeding
Abstract
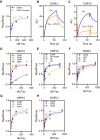
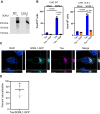
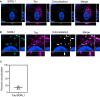
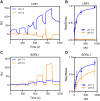
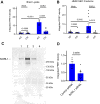
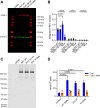
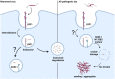
Sortilin-related receptor 1 (SORL1) is an intracellular sorting receptor genetically implicated in Alzheimer's disease (AD) that impacts amyloid precursor protein trafficking. The objective of these studies was to test the hypothesis that SORL1 binds tau, modulates its cellular trafficking and impacts the aggregation of cytoplasmic tau induced by pathological forms of tau. Using surface plasmon resonance measurements, we observed high-affinity binding of tau to SORL1 and the vacuolar protein sorting 10 domain of SORL1. Interestingly, unlike LDL receptor-related protein 1, SORL1 binds tau at both pH 7.4 and pH 5.5, revealing its ability to bind tau at endosomal pH. Immunofluorescence studies confirmed that exogenously added tau colocalized with SORL1 in H4 neuroglioma cells, while overexpression of SORL1 in LDL receptor-related protein 1-deficient Chinese hamster ovary (CHO) cells resulted in a marked increase in the internalization of tau, indicating that SORL1 can bind and mediate the internalization of monomeric forms of tau. We further demonstrated that SORL1 mediates tau seeding when tau RD P301S FRET biosensor cells expressing SORL1 were incubated with high molecular weight forms of tau isolated from the brains of patients with AD. Seeding in H4 neuroglioma cells is significantly reduced when SORL1 is knocked down with siRNA. Finally, we demonstrate that the N1358S mutant of SORL1 significantly increases tau seeding when compared to WT SORL1, identifying for the first time a potential mechanism that connects this specific SORL1 mutation to Alzheimer's disease. Together, these studies identify SORL1 as a receptor that contributes to trafficking and seeding of pathogenic tau.
Keywords: SORL1; apolipoprotein E (ApoE); lipoprotein receptor-related protein (LRP); neurodegenerative disease; tau protein (tau); tau seeding; tauopathy.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

