Biologic agents licensed for severe asthma: a systematic review and meta-analysis of randomised controlled trials
- PMID: 38657997
- PMCID: PMC11040390
- DOI: 10.1183/16000617.0238-2023
Biologic agents licensed for severe asthma: a systematic review and meta-analysis of randomised controlled trials
Abstract
Background: Six biologic agents are now approved for patients with severe asthma. This meta-analysis aimed to assess the efficacy and safety of licensed biologic agents in patients with severe asthma, including the recently approved tezepelumab.
Methods: We searched MEDLINE, Embase and CENTRAL to identify randomised controlled trials involving licensed biologics until 31 January 2023. We used random-effects meta-analysis models for efficacy, including subgroup analyses by individual agents and markers of T2-high inflammation (blood eosinophils and fractional exhaled nitric oxide), and assessed safety.
Results: 48 studies with 16 350 patients were included in the meta-analysis. Biologics were associated with a 44% reduction in the annualised rate of asthma exacerbations (rate ratio 0.56, 95% CI 0.51-0.62) and 60% reduction of hospitalisations (rate ratio 0.40, 95% CI 0.27-0.60), a mean increase in the forced expiratory volume in 1 s of 0.11 L (95% CI 0.09-0.14), a reduction in asthma control questionnaire by 0.34 points (95% CI -0.46--0.23) and an increase in asthma quality of life questionnaire by 0.38 points (95% CI 0.26-0.49). There was heterogeneity between different classes of biologics in certain outcomes, with overall greater efficacy in patients with T2 inflammation. Overall, biologics exhibited a favourable safety profile.
Conclusions: This comprehensive meta-analysis demonstrated that licensed asthma biologics reduce exacerbations and hospitalisations, improve lung function, asthma control and quality of life, and limit the use of systemic corticosteroids, with a favourable safety profile. These effects are more prominent in patients with evidence of T2 inflammation.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: A. Gogali has received consulting fees from Boehringer Ingelheim and Chiesi; and payment or honoraria for lectures, presentations or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK and Novartis. K. Kostikas has received grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Innovis, ELPEN, GSK, Menarini, Novartis and NuvoAir; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, ELPEN, GSK, Menarini, Novartis, Pfizer and Sanofi Genzyme; and payment or honoraria for lectures, presentations or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, ELPEN, GSK, Menarini, Novartis, Pfizer and Sanofi Genzyme. K. Kostikas is a member of the GOLD Assembly. C. Kyriakopoulos and G. Markozannes declare no competing interests.
Figures
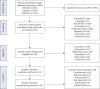


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Anti-interleukin-13 and anti-interleukin-4 agents versus placebo, anti-interleukin-5 or anti-immunoglobulin-E agents, for people with asthma.Cochrane Database Syst Rev. 2021 Oct 19;10(10):CD012929. doi: 10.1002/14651858.CD012929.pub2. Cochrane Database Syst Rev. 2021. PMID: 34664263 Free PMC article.
-
Anti-IL5 therapies for asthma.Cochrane Database Syst Rev. 2017 Sep 21;9(9):CD010834. doi: 10.1002/14651858.CD010834.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jul 12;7:CD010834. doi: 10.1002/14651858.CD010834.pub4. PMID: 28933516 Free PMC article. Updated.
-
Anti-IL-5 therapies for asthma.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD010834. doi: 10.1002/14651858.CD010834.pub4. Cochrane Database Syst Rev. 2022. PMID: 35838542 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Real-World Efficacy of Biological Therapies in Severe Asthma: A Focus on Small Airways.J Clin Med. 2024 Oct 2;13(19):5883. doi: 10.3390/jcm13195883. J Clin Med. 2024. PMID: 39407945 Free PMC article.
-
Anti-inflammatory reliever therapy (AIR) for asthma.ERJ Open Res. 2024 Sep 30;10(5):00494-2024. doi: 10.1183/23120541.00494-2024. eCollection 2024 Sep. ERJ Open Res. 2024. PMID: 39351389 Free PMC article.
-
Efficacy of Biologic Therapies in the Management of Allergic Rhinitis: A Systematic Review.Cureus. 2024 Oct 14;16(10):e71408. doi: 10.7759/cureus.71408. eCollection 2024 Oct. Cureus. 2024. PMID: 39539920 Free PMC article. Review.
-
Remission in Children Receiving Biologic Therapy for Severe Asthma.Pediatr Pulmonol. 2025 Aug;60(8):e71240. doi: 10.1002/ppul.71240. Pediatr Pulmonol. 2025. PMID: 40811151 Free PMC article. No abstract available.
-
Eosinophil-Driven vs. Eosinophil-Associated Severe Asthma: Practical Implications for Target Treatment.Int J Mol Sci. 2025 Feb 18;26(4):1729. doi: 10.3390/ijms26041729. Int J Mol Sci. 2025. PMID: 40004192 Free PMC article. Review.
References
-
- Global Initiative for Asthma . 2023 GINA Main Report. Date last accessed: 18 September 2023. Date last updated: 10 July 2023. https://ginasthma.org/2023-gina-main-report/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
