Safety of the PCSK9 inhibitor alirocumab: insights from 47 296 patient-years of observation
- PMID: 38658193
- PMCID: PMC11249957
- DOI: 10.1093/ehjcvp/pvae025
Safety of the PCSK9 inhibitor alirocumab: insights from 47 296 patient-years of observation
Abstract
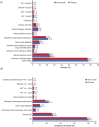
The ODYSSEY OUTCOMES trial, comprising over 47 000 patient-years of placebo-controlled observation, demonstrated important reductions in the risk of recurrent ischaemic cardiovascular events with the monoclonal antibody to proprotein convertase subtilisin/kexin type 9 alirocumab, as well as lower all-cause death. These benefits were observed in the context of substantial and persistent lowering of low-density lipoprotein cholesterol with alirocumab compared with that achieved with placebo. The safety profile of alirocumab was indistinguishable from matching placebo except for a ∼1.7% absolute increase in local injection site reactions. Further, the safety of alirocumab compared with placebo was evident in vulnerable groups identified before randomization, such as the elderly and those with diabetes mellitus, previous ischaemic stroke, or chronic kidney disease. The frequency of adverse events and laboratory-based abnormalities was generally similar to that in placebo-treated patients. Thus, alirocumab appears to be a safe and effective lipid-modifying treatment over a duration of at least 5 years.
Keywords: Alirocumab; Cholesterol; PCSK9; Safety.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 2012;366:1108–1118. - PubMed
-
- Roth EM, Mckenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–1900. - PubMed
-
- Mckenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand A-C, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344. - PubMed
-
- Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a Phase 2 randomised controlled trial. Lancet 2012;380:29–36. - PubMed
-
- Roth EM, Taskinen M-R, Ginsberg HN, Kastelein JJP, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24-week, double-blind, randomized Phase 3 trial. Int J Cardiol 2014;176:55–61. - PubMed

