Bacillus velezensis iturins inhibit the hemolytic activity of Staphylococcus aureus
- PMID: 38658583
- PMCID: PMC11043418
- DOI: 10.1038/s41598-024-58973-0
Bacillus velezensis iturins inhibit the hemolytic activity of Staphylococcus aureus
Abstract
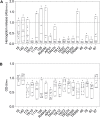
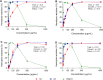
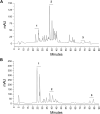
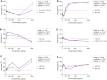
Bovine mastitis caused by S. aureus has a major economic impact on the dairy sector. With the crucial need for new therapies, anti-virulence strategies have gained attention as alternatives to antibiotics. Here we aimed to identify novel compounds that inhibit the production/activity of hemolysins, a virulence factor of S. aureus associated with mastitis severity. We screened Bacillus strains obtained from diverse sources for compounds showing anti-hemolytic activity. Our results demonstrate that lipopeptides produced by Bacillus spp. completely prevented the hemolytic activity of S. aureus at certain concentrations. Following purification, both iturins, fengycins, and surfactins were able to reduce hemolysis caused by S. aureus, with iturins showing the highest anti-hemolytic activity (up to 76% reduction). The lipopeptides showed an effect at the post-translational level. Molecular docking simulations demonstrated that these compounds can bind to hemolysin, possibly interfering with enzyme action. Lastly, molecular dynamics analysis indicated general stability of important residues for hemolysin activity as well as the presence of hydrogen bonds between iturins and these residues, with longevous interactions. Our data reveals, for the first time, an anti-hemolytic activity of lipopeptides and highlights the potential application of iturins as an anti-virulence therapy to control bovine mastitis caused by S. aureus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

