Elexacaftor/tezacaftor/ivacaftor influences body composition in adults with cystic fibrosis: a fully automated CT-based analysis
- PMID: 38658613
- PMCID: PMC11043331
- DOI: 10.1038/s41598-024-59622-2
Elexacaftor/tezacaftor/ivacaftor influences body composition in adults with cystic fibrosis: a fully automated CT-based analysis
Abstract
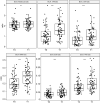
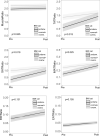
A poor nutritional status is associated with worse pulmonary function and survival in people with cystic fibrosis (pwCF). CF transmembrane conductance regulator modulators can improve pulmonary function and body weight, but more data is needed to evaluate its effects on body composition. In this retrospective study, a pre-trained deep-learning network was used to perform a fully automated body composition analysis on chest CTs from 66 adult pwCF before and after receiving elexacaftor/tezacaftor/ivacaftor (ETI) therapy. Muscle and adipose tissues were quantified and divided by bone volume to obtain body size-adjusted ratios. After receiving ETI therapy, marked increases were observed in all adipose tissue ratios among pwCF, including the total adipose tissue ratio (+ 46.21%, p < 0.001). In contrast, only small, but statistically significant increases of the muscle ratio were measured in the overall study population (+ 1.63%, p = 0.008). Study participants who were initially categorized as underweight experienced more pronounced effects on total adipose tissue ratio (p = 0.002), while gains in muscle ratio were equally distributed across BMI categories (p = 0.832). Our findings suggest that ETI therapy primarily affects adipose tissues, not muscle tissue, in adults with CF. These effects are primarily observed among pwCF who were initially underweight. Our findings may have implications for the future nutritional management of pwCF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J. Am. Diet. Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

