Dynamic control of gene expression by ISGF3 and IRF1 during IFNβ and IFNγ signaling
- PMID: 38658796
- PMCID: PMC11148166
- DOI: 10.1038/s44318-024-00092-7
Dynamic control of gene expression by ISGF3 and IRF1 during IFNβ and IFNγ signaling
Abstract
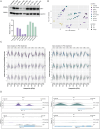
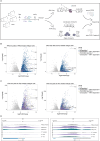
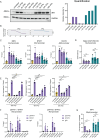
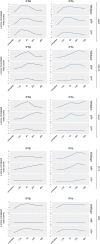
Type I interferons (IFN-I, including IFNβ) and IFNγ produce overlapping, yet clearly distinct immunological activities. Recent data show that the distinctness of global transcriptional responses to the two IFN types is not apparent when comparing their immediate effects. By analyzing nascent transcripts induced by IFN-I or IFNγ over a period of 48 h, we now show that the distinctiveness of the transcriptomes emerges over time and is based on differential employment of the ISGF3 complex as well as of the second-tier transcription factor IRF1. The distinct transcriptional properties of ISGF3 and IRF1 correspond with a largely diverse nuclear protein interactome. Mechanistically, we describe the specific input of ISGF3 and IRF1 into enhancer activation and the regulation of chromatin accessibility at interferon-stimulated genes (ISG). We further report differences between the IFN types in altering RNA polymerase II pausing at ISG 5' ends. Our data provide insight how transcriptional regulators create immunological identities of IFN-I and IFNγ.
Keywords: IRF; Interferons; Macrophage; STAT; Transcription.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

