Cost-effectiveness of sacituzumab govitecan versus single-agent chemotherapy for metastatic triple-negative breast cancer: a trial-based analysis
- PMID: 38659013
- PMCID: PMC11044338
- DOI: 10.1186/s12962-024-00539-y
Cost-effectiveness of sacituzumab govitecan versus single-agent chemotherapy for metastatic triple-negative breast cancer: a trial-based analysis
Abstract
Background: Sacituzumab govitecan (SG) has recently been approved in China for the post-line treatment of metastatic triple-negative breast cancer (mTNBC). SG substantially improves progression-free survival and overall survival compared with single-agent chemotherapy for pretreated mTNBC. However, in view of the high price of SG, it is necessary to consider its value in terms of costs and outcomes. This study aimed to estimate the cost-effectiveness of SG versus single-agent treatment of physician's choice (TPC) in the post-line setting for patients with mTNBC from a Chinese healthcare system perspective.
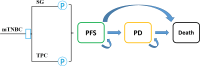
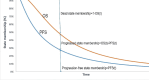
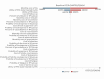
Methods: The cohort characteristics were sourced from the ASCENT randomized clinical trial, which enrolled 468 heavily pretreated patients with mTNBC between November 2017 and September 2019. A partitioned survival model was constructed to assess the long-term costs and effectiveness of SG versus TPC in the post-line treatment of mTNBC. Quality-adjusted life-months (QALMs) and total costs in 2022 US dollars were used to derive incremental cost effectiveness ratio (ICER). QALMs and costs were discounted at 5% annually. The willingness-to-pay (WTP) threshold was defined as $3188 per QALM, three times China's average monthly per capita gross domestic product in 2022. One-way sensitivity analysis, probabilistic sensitivity analysis, and scenario analyses were performed to estimate the robustness of the results.
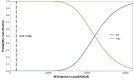
Results: Treatment with SG yielded an incremental 5.17 QALMs at a cost of $44,792 per QALM, much above the WTP threshold of $3188/QALM in China. One-way sensitivity analysis showed that SG price was a crucial factor in the ICER. Probabilistic sensitivity analysis revealed that the cost-effective acceptability of SG was 0% in the current setting. Scenario analyses indicated that the result was robust in all subgroups in ASCENT or under different time horizons. Furthermore, SG must reduce the price to enter the Chinese mainland market. When the monthly cost of SG reduce to $2298, SG has about 50% probability to be a preferred choice than TPC.
Conclusions: SG was estimated to be not cost-effective compared with TPC for post-line treatment for mTNBC in China by the current price in HK under a WTP threshold of $3188 per QALM. A drastic price reduction is necessary to improve its cost-effectiveness.
Keywords: Cost-effectiveness analysis; Metastatic triple-negative breast cancer; Sacituzumab govitecan; Single-agent chemotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cost-effectiveness of sacituzumab govitecan for hormone receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer based on the EVER-132-002 trial in China.Cost Eff Resour Alloc. 2025 Mar 19;23(1):8. doi: 10.1186/s12962-025-00613-z. Cost Eff Resour Alloc. 2025. PMID: 40108623 Free PMC article.
-
Cost-effectiveness of Sacituzumab Govitecan versus Single-agent Chemotherapy for Patients with Metastatic Triple-Negative Breast Cancer in China.Clin Breast Cancer. 2024 Oct;24(7):e545-e553.e6. doi: 10.1016/j.clbc.2024.04.010. Epub 2024 Apr 23. Clin Breast Cancer. 2024. PMID: 38760263
-
Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US.Front Oncol. 2021 Oct 28;11:734594. doi: 10.3389/fonc.2021.734594. eCollection 2021. Front Oncol. 2021. PMID: 34778047 Free PMC article.
-
Sacituzumab govitecan: past, present and future of a new antibody-drug conjugate and future horizon.Future Oncol. 2022 Sep;18(28):3199-3215. doi: 10.2217/fon-2022-0407. Epub 2022 Sep 7. Future Oncol. 2022. PMID: 36069628 Review.
-
The European Medicines Agency review of sacituzumab govitecan for the treatment of triple-negative breast cancer.ESMO Open. 2022 Jun;7(3):100497. doi: 10.1016/j.esmoop.2022.100497. Epub 2022 May 25. ESMO Open. 2022. PMID: 35642987 Free PMC article. Review.
Cited by
-
Cost-effectiveness of sacituzumab govitecan for hormone receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer based on the EVER-132-002 trial in China.Cost Eff Resour Alloc. 2025 Mar 19;23(1):8. doi: 10.1186/s12962-025-00613-z. Cost Eff Resour Alloc. 2025. PMID: 40108623 Free PMC article.
References
-
- Chai J, Hu J, Wang T, Bao X, Luan J, Wang Y. A multifunctional liposome for synergistic chemotherapy with ferroptosis activation of Triple-negative breast Cancer. Mol Pharm. 2024;21:781–90. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. - PubMed
-
- Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-negative breast Cancer. N Engl J Med. 2022;387:217–26. - PubMed
Grants and funding
- Z-2021-46-2101/Research Fund for Clinical Pharmacy of China International Medical Foundation
- Z-2021-46-2101/Research Fund for Clinical Pharmacy of China International Medical Foundation
- 82003844/National Natural Science Foundation of China
- SHWSRS (2020) 087/Shanghai "Rising Stars of Medical Talents" Youth Development Program-Youth Medical Talents: Clinical Pharmacist Program
LinkOut - more resources
Full Text Sources

