Coordination of COVID-19 platform trials in Europe
- PMID: 38659031
- PMCID: PMC11044280
- DOI: 10.1186/s13063-024-08126-5
Coordination of COVID-19 platform trials in Europe
Abstract
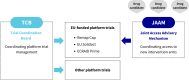
To ensure optimal coordination of the EU-funded COVID-19 platform trials, a double coordination mechanism was established. It included the Trial Coordination Board (TCB) to promote the dialogue between investigators and relevant public health stakeholders and the Joint Access Advisory Mechanism (JAAM) to streamline access of new intervention arms to the platform trials. Both the TCB and the JAAM emerged as efficient instruments to promote cooperation and optimise the use of resources within EU-funded adaptive platform trials. In addition, an adaptive platform trial toolbox was developed to collect information and literature on challenges and solutions identified to date. The recently funded 'Coordination MEchanism for Cohorts and Trials' (CoMeCT) project will endeavour to make this model sustainable, with a further expansion to other emerging infectious diseases, as part of the governance of the current and future platform trials for pandemic preparedness. This example could serve as a model for platform trial coordination in other disease areas.
Keywords: Adaptive platform trial; COVID-19; Coordination mechanism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- European Medicines Agency. Call to pool research resources into large multi-centre, multi-arm clinical trials to generate sound evidence on COVID-19 treatments. 2020. https://www.ema.europa.eu/en/news/call-pool-research-resources-large-mul... .Accessed 31Mar 2022.
-
- Mullard A. COVID-19 platform trial delivers. Nat Rev Drug Discov. 2020;19:501. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

