Mendelian randomization and colocalization analysis reveal novel drug targets for myasthenia gravis
- PMID: 38659056
- PMCID: PMC11040902
- DOI: 10.1186/s40246-024-00607-7
Mendelian randomization and colocalization analysis reveal novel drug targets for myasthenia gravis
Abstract
Objective: Myasthenia gravis (MG) is a complex autoimmune disease affecting the neuromuscular junction with limited drug options, but the field of MG treatment recently benefits from novel biological agents. We performed a drug-targeted Mendelian randomization (MR) study to identify novel therapeutic targets of MG.
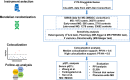
Methods: Cis-expression quantitative loci (cis-eQTL), which proxy expression levels for 2176 druggable genes, were used for MR analysis. Causal relationships between genes and disease, identified by eQTL MR analysis, were verified by comprehensive sensitivity, colocalization, and protein quantitative loci (pQTL) MR analyses. The protein-protein interaction (PPI) analysis was also performed to extend targets, followed by enzyme-linked immunosorbent assay (ELISA) to explore the serum level of drug targets in MG patients. A phenome-wide MR analysis was then performed to assess side effects with a clinical trial review assessing druggability.
Results: The eQTL MR analysis has identified eight potential targets for MG, one for early-onset MG and seven for late-onset MG. Further colocalization analyses indicated that CD226, CDC42BPB, PRSS36, and TNFSF12 possess evidence for colocalization with MG or late-onset MG. pQTL MR analyses identified the causal relations of TNFSF12 and CD226 with MG and late-onset MG. Furthermore, PPI analysis has revealed the protein interaction between TNFSF12-TNFSF13(APRIL) and TNFSF12-TNFSF13B(BLyS). Elevated TNFSF13 serum level of MG patients was also identified by ELISA experiments. This study has ultimately proposed three promising therapeutic targets (TNFSF12, TNFSF13, TNFSF13B) of MG.
Conclusions: Three drug targets associated with the BLyS/APRIL pathway have been identified. Multiple biological agents, including telitacicept and belimumab, are promising for MG therapy.
Keywords: BLyS/APRIL pathway; Colocalization analysis; Drug target; Mendelian randomization study; Myasthenia gravis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Integrative multi-omics analysis identifies genetically supported druggable targets and immune cell specificity for myasthenia gravis.J Transl Med. 2024 Mar 24;22(1):302. doi: 10.1186/s12967-024-04994-2. J Transl Med. 2024. PMID: 38521921 Free PMC article.
-
Multi-omic studies on the pathogenesis of Sepsis.J Transl Med. 2025 Mar 24;23(1):361. doi: 10.1186/s12967-025-06366-w. J Transl Med. 2025. PMID: 40128726 Free PMC article.
-
Mendelian randomization analysis identifies druggable genes and drugs repurposing for chronic obstructive pulmonary disease.Front Cell Infect Microbiol. 2024 Apr 10;14:1386506. doi: 10.3389/fcimb.2024.1386506. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38660492 Free PMC article.
-
Serum Inflammatory Factors Levels and Risk of Myasthenia Gravis: A Bidirectional Mendelian Randomization Study.Mol Neurobiol. 2025 Jun;62(6):7738-7746. doi: 10.1007/s12035-025-04744-5. Epub 2025 Feb 11. Mol Neurobiol. 2025. PMID: 39932515
-
Genetic Insights into Therapeutic Targets for Neuromyelitis Optica Spectrum Disorders: A Mendelian Randomization Study.Mol Neurobiol. 2025 May;62(5):5518-5530. doi: 10.1007/s12035-024-04612-8. Epub 2024 Nov 20. Mol Neurobiol. 2025. PMID: 39565569
Cited by
-
Circulating inflammatory proteins as pathogenic mediators and potential therapeutic targets in myasthenia gravis.Neurol Sci. 2025 Jun 26. doi: 10.1007/s10072-025-08328-y. Online ahead of print. Neurol Sci. 2025. PMID: 40569533
-
Advances in the genetics of myasthenia gravis: insights from cutting-edge neuroscience research.Front Med (Lausanne). 2025 Jan 7;11:1508422. doi: 10.3389/fmed.2024.1508422. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39845831 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

