Circular RNA vaccines against monkeypox virus provide potent protection against vaccinia virus infection in mice
- PMID: 38659224
- PMCID: PMC11184329
- DOI: 10.1016/j.ymthe.2024.04.028
Circular RNA vaccines against monkeypox virus provide potent protection against vaccinia virus infection in mice
Abstract
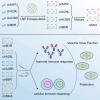
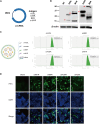
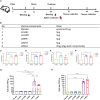
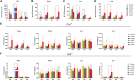
Since the outbreak of monkeypox (mpox) in 2022, widespread concern has been placed on imposing an urgent demand for specific vaccines that offer safer and more effective protection. Using an efficient and scalable circular RNA (circRNA) platform, we constructed four circRNA vaccines that could induce robust neutralizing antibodies as well as T cell responses by expressing different surface proteins of mpox virus (MPXV), resulting in potent protection against vaccinia virus (VACV) in mice. Strikingly, the combination of the four circular RNA vaccines demonstrated the best protection against VACV challenge among all the tested vaccines. Our study provides a favorable approach for developing MPXV-specific vaccines by using a circular mRNA platform and opens up novel avenues for future vaccine research.
Keywords: circular RNA vaccines; immune responses; mice; monkeypox virus; protection.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.Y., K.Z., J.H., and F.L. are employees of CirCode. Z.W. is an advisor to CirCode. Z.W. and Y.Y. are cofounders of CirCode Biomed. There is one unpublished patent application directed to the subject matter of the paper.
Figures
Similar articles
-
Long-Lasting Protection and Dose Optimization of MPXV Polyvalent Mpox mRNA Vaccines Against Lethal Vaccinia Virus Challenge in Mice.J Med Virol. 2025 Jan;97(1):e70143. doi: 10.1002/jmv.70143. J Med Virol. 2025. PMID: 39726255
-
Polyvalent mpox mRNA vaccines elicit robust immune responses and confer potent protection against vaccinia virus.Cell Rep. 2024 Jun 25;43(6):114269. doi: 10.1016/j.celrep.2024.114269. Epub 2024 May 23. Cell Rep. 2024. PMID: 38787725
-
A bivalent Mpox nanoparticle vaccine induces robust immune response and provides long-lasting protection against vaccinia virus challenge.Emerg Microbes Infect. 2025 Dec;14(1):2535485. doi: 10.1080/22221751.2025.2535485. Epub 2025 Jul 28. Emerg Microbes Infect. 2025. PMID: 40720261 Free PMC article.
-
Current Status of Vaccine Development for Monkeypox Virus.Adv Exp Med Biol. 2024;1451:289-300. doi: 10.1007/978-3-031-57165-7_18. Adv Exp Med Biol. 2024. PMID: 38801585 Review.
-
Mpox virus (MPXV): comprehensive analysis of pandemic risks, pathophysiology, treatments, and mRNA vaccine development.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6143-6163. doi: 10.1007/s00210-024-03649-9. Epub 2025 Jan 8. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39777535 Review.
Cited by
-
The Application of Non-Coding RNAs as Biomarkers, Therapies, and Novel Vaccines in Diseases.Int J Mol Sci. 2025 Mar 26;26(7):3055. doi: 10.3390/ijms26073055. Int J Mol Sci. 2025. PMID: 40243658 Free PMC article. Review.
-
A single-dose circular RNA vaccine prevents Zika virus infection without enhancing dengue severity in mice.Nat Commun. 2024 Oct 16;15(1):8932. doi: 10.1038/s41467-024-53242-0. Nat Commun. 2024. PMID: 39414822 Free PMC article.
-
Roles of circRNAs in viral pathogenesis.Front Cell Infect Microbiol. 2025 Mar 13;15:1564258. doi: 10.3389/fcimb.2025.1564258. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40182764 Free PMC article. Review.
-
Unlocking the potential of circular RNA vaccines: a bioinformatics and computational biology perspective.EBioMedicine. 2025 Apr;114:105638. doi: 10.1016/j.ebiom.2025.105638. Epub 2025 Mar 19. EBioMedicine. 2025. PMID: 40112741 Free PMC article. Review.
-
Rational Design and Immunological Mechanisms of Circular RNA-Based Vaccines: Emerging Frontiers in Combating Pathogen Infection.Vaccines (Basel). 2025 May 26;13(6):563. doi: 10.3390/vaccines13060563. Vaccines (Basel). 2025. PMID: 40573894 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

