A role for vessel-associated extracellular matrix proteins in multiple sclerosis pathology
- PMID: 38659387
- PMCID: PMC11483522
- DOI: 10.1111/bpa.13263
A role for vessel-associated extracellular matrix proteins in multiple sclerosis pathology
Abstract
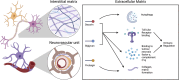
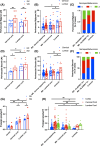
Multiple sclerosis (MS) is unsurpassed for its clinical and pathological hetherogeneity, but the biological determinants of this variability are unknown. HLA-DRB1*15, the main genetic risk factor for MS, influences the severity and distribution of MS pathology. This study set out to unravel the molecular determinants of the heterogeneity of MS pathology in relation to HLA-DRB1*15 status. Shotgun proteomics from a discovery cohort of MS spinal cord samples segregated by HLA-DRB*15 status revealed overexpression of the extracellular matrix (ECM) proteins, biglycan, decorin, and prolargin in HLA-DRB*15-positive cases, adding to established literature on a role of ECM proteins in MS pathology that has heretofore lacked systematic pathological validation. These findings informed a neuropathological characterisation of these proteins in a large autopsy cohort of 41 MS cases (18 HLA-DRB1*15-positive and 23 HLA-DRB1*15-negative), and seven non-neurological controls on motor cortical, cervical and lumbar spinal cord tissue. Biglycan and decorin demonstrate a striking perivascular expression pattern in controls that is reduced in MS (-36.5%, p = 0.036 and - 24.7%, p = 0.039; respectively) in lesional and non-lesional areas. A concomitant increase in diffuse parenchymal accumulation of biglycan and decorin is seen in MS (p = 0.015 and p = 0.001, respectively), particularly in HLA-DRB1*15-positive cases (p = 0.007 and p = 0.046, respectively). Prolargin shows a faint parenchymal pattern in controls that is markedly increased in MS cases where a perivascular deposition pattern is observed (motor cortex +97.5%, p = 0.001; cervical cord +49.1%, p = 0.016). Our findings point to ECM proteins and the vascular interface playing a central role in MS pathology within and outside the plaque area. As ECM proteins are known potent pro-inflammatory molecules, their parenchymal accumulation may contribute to disease severity. This study brings to light novel factors that may contribute to the heterogeneity of the topographical variation of MS pathology.
Keywords: ECM proteins; HLA‐DRB1*15; MS; pathology; topography; vessel.
© 2024 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25–36. - PubMed
-
- Brownlee WJ, Tur C, Manole A, Eshaghi A, Prados F, Miszkiel KA, et al. HLA‐DRB1*1501 influences long‐term disability progression and tissue damage on MRI in relapse‐onset multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2023;29(3):333–342. - PubMed
-
- DeLuca GC, Alterman R, Martin JL, Mittal A, Blundell S, Bird S, et al. Casting light on multiple sclerosis heterogeneity: the role of HLA‐DRB1 on spinal cord pathology. Brain J Neurol. 2013;136(Pt 4):1025–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

