This is a preprint.
Functional cardiac consequences of β-adrenergic stress-induced injury in the mdx mouse model of Duchenne muscular dystrophy
- PMID: 38659739
- PMCID: PMC11042272
- DOI: 10.1101/2024.04.15.589650
Functional cardiac consequences of β-adrenergic stress-induced injury in the mdx mouse model of Duchenne muscular dystrophy
Update in
-
Functional cardiac consequences of β-adrenergic stress-induced injury in a model of Duchenne muscular dystrophy.Dis Model Mech. 2024 Oct 1;17(10):dmm050852. doi: 10.1242/dmm.050852. Epub 2024 Oct 9. Dis Model Mech. 2024. PMID: 39268580 Free PMC article.
Abstract
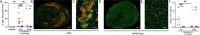
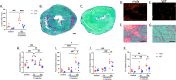
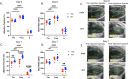
Cardiomyopathy is the leading cause of death in Duchenne muscular dystrophy (DMD), however, in the mdx mouse model of DMD, the cardiac phenotype differs from that seen in DMD-associated cardiomyopathy. Although some have used pharmacologic stress to enhance the cardiac phenotype in the mdx model, many methods lead to high mortality, variable cardiac outcomes, and do not recapitulate the structural and functional cardiac changes seen in human disease. Here, we describe a simple and effective method to enhance the cardiac phenotype model in mdx mice using advanced 2D and 4D high-frequency ultrasound to monitor cardiac dysfunction progression in vivo. For our study, mdx and wild-type (WT) mice received daily low-dose (2 mg/kg/day) isoproterenol injections for 10 days. Histopathologic assessment showed that isoproterenol treatment increased myocyte injury, elevated serum cardiac troponin I levels, and enhanced fibrosis in mdx mice. Ultrasound revealed reduced ventricular function, decreased wall thickness, increased volumes, and diminished cardiac reserve in mdx mice compared to wild-type. Our findings highlight the utility of low-dose isoproterenol in mdx mice as a valuable model for exploring therapies targeting DMD-associated cardiac complications.
Keywords: 4DUS; Duchenne muscular dystrophy; cardiac strain; isoproterenol; mdx; mouse model.
Conflict of interest statement
Declaration of Interest CJG is a paid consultant of FUJIFILM VisualSonics Inc. Declaration of generative AI and AI-assisted technologies in the writing process During the preparation of this work, the authors used ChatGPT(OpenAI, San Francisco, California) in some minor instances to rephrase and/or summarize text in order to improve readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Figures




References
-
- Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, Matthews DJ, Meaney FJ, Andrews JG, Conway KM, Fox DJ, Street N, Adams MM, Bolen J. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135(3):513–21. doi: 10.1542/peds.2014-2044. - DOI - PMC - PubMed
-
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. - PubMed
-
- Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347–61. doi: 10.1016/s1474-4422(18)30025-5. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
