This is a preprint.
CD4-mediated immunity shapes neutrophil-driven tuberculous pathology
- PMID: 38659794
- PMCID: PMC11042216
- DOI: 10.1101/2024.04.12.589315
CD4-mediated immunity shapes neutrophil-driven tuberculous pathology
Update in
-
Early and opposing neutrophil and CD4 T cell responses shape pulmonary tuberculosis pathology.J Exp Med. 2025 Oct 6;222(10):e20250161. doi: 10.1084/jem.20250161. Epub 2025 Jul 30. J Exp Med. 2025. PMID: 40736456 Free PMC article.
Abstract
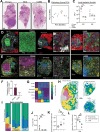
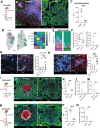
Pulmonary Mycobacterium tuberculosis (Mtb) infection results in highly heterogeneous lesions ranging from granulomas with central necrosis to those primarily comprised of alveolitis. While alveolitis has been associated with prior immunity in human post-mortem studies, the drivers of these distinct pathologic outcomes are poorly understood. Here, we show that these divergent lesion structures can be modeled in C3HeB/FeJ mice and are regulated by prior immunity. Using quantitative imaging, scRNAseq, and flow cytometry, we demonstrate that Mtb infection in the absence of prior immunity elicits dysregulated neutrophil recruitment and necrotic granulomas. In contrast, prior immunity induces rapid recruitment and activation of T cells, local macrophage activation, and diminished late neutrophil responses. Depletion studies at distinct infection stages demonstrated that neutrophils are required for early necrosis initiation and necrosis propagation at chronic stages, whereas early CD4 T cell responses prevent neutrophil feedforward circuits and necrosis. Together, these studies reveal fundamental determinants of tuberculosis lesion structure and pathogenesis, which have important implications for new strategies to prevent or treat tuberculosis.
Keywords: CD4 T cells; Mycobacterium tuberculosis; alveolitis; granuloma; neutrophils; quantitative imaging; tuberculosis.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Rich A.R. (1944). The Pathogenesis of Tuberculosis (C. C. Thomas; ).
-
- Gautam U.S., Foreman T.W., Bucsan A.N., Veatch A.V., Alvarez X., Adekambi T., Golden N.A., Gentry K.M., Doyle-Meyers L.A., Russell-Lodrigue K.E., et al. (2017). In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci., 201711373. 10.1073/pnas.1711373114. - DOI - PMC - PubMed
-
- Kauffman K.D., Sallin M.A., Sakai S., Kamenyeva O., Kabat J., Weiner D., Sutphin M., Schimel D., Via L., Barry C.E., et al. (2017). Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol. 10.1038/mi.2017.60. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
