This is a preprint.
Polycystins recruit cargo to distinct ciliary extracellular vesicle subtypes
- PMID: 38659811
- PMCID: PMC11042387
- DOI: 10.1101/2024.04.17.588758
Polycystins recruit cargo to distinct ciliary extracellular vesicle subtypes
Update in
-
Polycystins recruit cargo to distinct ciliary extracellular vesicle subtypes in C. elegans.Nat Commun. 2025 Apr 3;16(1):2899. doi: 10.1038/s41467-025-57512-3. Nat Commun. 2025. PMID: 40180912 Free PMC article.
Abstract
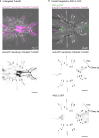
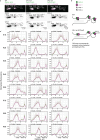
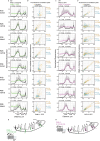
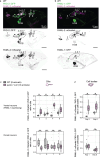
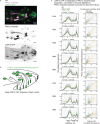
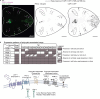
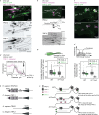
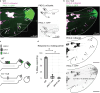
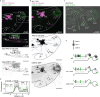
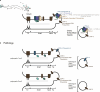
Therapeutic use of tiny extracellular vesicles (EVs) requires understanding cargo loading mechanisms. Here, we used a modular proximity label approach to identify EV cargo associated with the transient potential channel (TRP) polycystin PKD-2 of C. elegans. Polycystins are conserved receptor-TRP channel proteins affecting cilium function; dysfunction causes polycystic kidney disease in humans and mating deficits in C. elegans. Polycystin-2 EV localization is conserved from algae to humans, hinting at an ancient and unknown function. We discovered that polycystins associate with and direct specific cargo to EVs: channel-like PACL-1, dorsal and ventral membrane C-type lectins PAMLs, and conserved tumor necrosis-associated factor (TRAF) signaling adaptors TRF-1 and TRF-2. Loading of these components relied on polycystin-1 LOV-1. Our modular EV-TurboID approach can be applied in both cell- and tissue-specific manners to define the composition of distinct EV subtypes, addressing a major challenge of the EV field.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures







References
-
- Wyart C., Carbo-Tano M., Cantaut-Belarif Y., Orts-Del’Immagine A. & Böhm U. L. Cerebrospinal fluid-contacting neurons: multimodal cells with diverse roles in the CNS. Nat Rev Neurosci 24, 540–556 (2023). - PubMed
-
- Orts-Del’Immagine A. et al. Sensory Neurons Contacting the Cerebrospinal Fluid Require the Reissner Fiber to Detect Spinal Curvature In Vivo. Current Biology 30, 827–839.e4 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
