This is a preprint.
Influenza virus antibodies inhibit antigen-specific de novo B cell responses in mice
- PMID: 38659819
- PMCID: PMC11042189
- DOI: 10.1101/2024.04.12.589218
Influenza virus antibodies inhibit antigen-specific de novo B cell responses in mice
Update in
-
Influenza virus antibodies inhibit antigen-specific de novo B cell responses in mice.J Virol. 2024 Sep 17;98(9):e0076624. doi: 10.1128/jvi.00766-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194245 Free PMC article.
Abstract
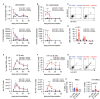
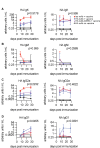
Antibody responses to influenza vaccines tend to be focused on epitopes encountered during prior influenza exposures, with little production of de novo responses to novel epitopes. To examine the contribution of circulating antibody to this phenomenon, we passively transferred a hemagglutinin (HA)-specific monoclonal antibody (mAb) into mice before immunizing with whole inactivated virions. The HA mAb inhibited de novo HA-specific antibodies, plasmablasts, germinal center B cells, and memory B cells, while responses to a second antigen in the vaccine, neuraminidase (NA), were uninhibited. The HA mAb potently inhibited de novo antibody responses against epitopes near the HA mAb binding site. The HA mAb also promoted IgG1 class switching, an effect that, unlike the inhibition of HA responses, relied on signaling through Fc-gamma receptors. These studies suggest that circulating antibodies inhibit de novo B cell responses in an antigen-specific manner, which likely contributes to differences in antibody specificities elicited during primary and secondary influenza virus exposures.
Conflict of interest statement
Declaration of Interests S.E.H. is a co-inventors on patents that describe the use of nucleoside-modified mRNA as a vaccine platform. S.E.H reports receiving consulting fees from Sanofi, Pfizer, Lumen, Novavax, and Merck.
Figures


Similar articles
-
Influenza virus antibodies inhibit antigen-specific de novo B cell responses in mice.J Virol. 2024 Sep 17;98(9):e0076624. doi: 10.1128/jvi.00766-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194245 Free PMC article.
-
Alternative recognition of the conserved stem epitope in influenza A virus hemagglutinin by a VH3-30-encoded heterosubtypic antibody.J Virol. 2014 Jun;88(12):7083-92. doi: 10.1128/JVI.00178-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719426 Free PMC article.
-
Inactivated influenza virions are a flexible vaccine platform for eliciting protective antibody responses against neuraminidase.Vaccine. 2023 Jun 29;41(29):4302-4312. doi: 10.1016/j.vaccine.2023.05.068. Epub 2023 Jun 8. Vaccine. 2023. PMID: 37301705
-
Influenza vaccine immunology.Immunol Rev. 2011 Jan;239(1):167-77. doi: 10.1111/j.1600-065X.2010.00974.x. Immunol Rev. 2011. PMID: 21198671 Review.
-
Role of Memory B Cells in Hemagglutinin-Specific Antibody Production Following Human Influenza A Virus Infection.Pathogens. 2019 Sep 28;8(4):167. doi: 10.3390/pathogens8040167. Pathogens. 2019. PMID: 31569328 Free PMC article. Review.
References
-
- Bandukwala H. S., Clay B. S., Tong J., Mody P. D., Cannon J. L., Shilling R. A., Verbeek J. S., Weinstock J. V., Solway J., & Sperling A. I. (2007). Signaling through FcγRIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. Journal of Experimental Medicine, 204(8), 1875–1889. 10.1084/jem.20061134 - DOI - PMC - PubMed
-
- Baudino L., Shinohara Y., Nimmerjahn F., Furukawa J.-I., Nakata M., Martínez-Soria E., Petry F., Ravetch J. V., Nishimura S.-I., & Izui S. (2008). Crucial Role of Aspartic Acid at Position 265 in the CH2 Domain for Murine IgG2a and IgG2b Fc-Associated Effector Functions. The Journal of Immunology, 181(9), 6664–6669. 10.4049/jimmunol.181.9.6664 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
